Structure-switching M3L2 Ir(iii) coordination cages with photo-isomerising azo-aromatic linkers
- PMID: 30542566
- PMCID: PMC6238882
- DOI: 10.1039/c8sc03499k
Structure-switching M3L2 Ir(iii) coordination cages with photo-isomerising azo-aromatic linkers
Abstract
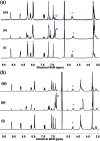
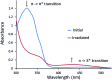
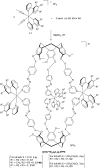
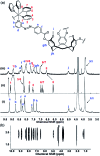
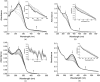
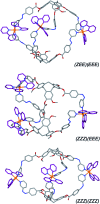
Cyclotriguaiacylene has been functionalised with 3- or 4-pyridyl-azo-phenyl groups to form a series of molecular hosts with three azobenzene-type groups that exhibit reversible photo-isomerisation. Reaction of the host molecules with [Ir(C^N)2(NCMe)2]+ where C^N is the cyclometallating 2-phenylpyridinato, 2-(4-methylphenyl)pyridinato or 2-(4,5,6-trifluorophenyl)pyridinato results in the self-assembly of a family of five different [{Ir(C^N)2}3(L)2]3+ coordination cages. Photo-irradiation of each of the cages with a high energy laser results in E → Z photo-isomerisation of the pyridyl-azo-phenyl groups with up to 40% of groups isomerising. Isomerisation can be reversed by exposure to blue light. Thus, the cages show reversible structure-switching while maintaining their compositional integrity. This represents the largest photo-induced structural change yet reported for a structurally-integral component of a coordination cage. Energy minimised molecular models indicate a switched cage has a smaller internal space than the initial all-E isomer. The [Ir(C^N)2(NCMe)2]+ cages are weakly emissive, each with a deep blue luminescence at ca. 450 nm.
Figures






Similar articles
-
Homochiral Self-Sorted and Emissive IrIII Metallo-Cryptophanes.Chemistry. 2017 May 5;23(26):6290-6294. doi: 10.1002/chem.201701348. Epub 2017 Apr 20. Chemistry. 2017. PMID: 28370620 Free PMC article.
-
Cation-Anion Arrangement Patterns in Self-Assembled Pd2L4 and Pd4L8 Coordination Cages.Acc Chem Res. 2017 Sep 19;50(9):2233-2243. doi: 10.1021/acs.accounts.7b00231. Epub 2017 Aug 17. Acc Chem Res. 2017. PMID: 28817257
-
Light-Triggered Reversible Open-Close Motion of a Chiral Molecular Plier to Modulate Guest Binding.Chemistry. 2023 May 16;29(28):e202300092. doi: 10.1002/chem.202300092. Epub 2023 Mar 31. Chemistry. 2023. PMID: 36872293
-
Polyanionic Imido-P(V) Ligands: From Transition Metal Complexes to Coordination Driven Self-Assemblies.Chem Rec. 2022 Mar;22(3):e202100281. doi: 10.1002/tcr.202100281. Epub 2021 Dec 27. Chem Rec. 2022. PMID: 34962082 Review.
-
Photo-mechanical effects in azobenzene-containing soft materials.Soft Matter. 2007 Sep 19;3(10):1249-1261. doi: 10.1039/b705619b. Soft Matter. 2007. PMID: 32900091 Review.
Cited by
-
Adaptive coordination assemblies based on a flexible tetraazacyclododecane ligand for promoting carbon dioxide fixation.Chem Sci. 2022 Jul 5;13(31):9016-9022. doi: 10.1039/d2sc03093d. eCollection 2022 Aug 10. Chem Sci. 2022. PMID: 36091216 Free PMC article.
-
Conformer-dependent self-assembled metallacycles with photo-reversible response.Chem Sci. 2019 Mar 25;10(18):4896-4904. doi: 10.1039/c9sc00757a. eCollection 2019 May 14. Chem Sci. 2019. PMID: 31160961 Free PMC article.
-
Light-Driven Purification of Progesterone from Steroid Mixtures Using a Photoresponsive Metal-Organic Capsule.J Am Chem Soc. 2024 Jan 31;146(4):2568-2573. doi: 10.1021/jacs.3c11005. Epub 2024 Jan 17. J Am Chem Soc. 2024. PMID: 38230667 Free PMC article.
-
Visible-Light Switching of Metallosupramolecular Assemblies.Chemistry. 2022 Mar 16;28(16):e202104461. doi: 10.1002/chem.202104461. Epub 2022 Feb 19. Chemistry. 2022. PMID: 35102616 Free PMC article.
-
Conformation-Driven Self-Assembly: From a 1D Metal-Organic Polymer to an Infinite Double Nanotube.ACS Omega. 2019 Jun 20;4(6):10755-10760. doi: 10.1021/acsomega.9b01344. eCollection 2019 Jun 30. ACS Omega. 2019. PMID: 31460173 Free PMC article.
References
-
- Reviews: Chen L.-J., Yang H.-B., Shionoya M., Chem. Soc. Rev., 2017, 46 , 2555 Bloch W. M., Clever G. H., Chem. Commun., 2017, 53 , 8506, D Ward M., Hunter C. A., Williams N. H., Chem. Lett., 2017, 46 , 2, Vasdev R. A. S., Preston D., Crowley J. D., Chem.–Asian J., 2017, 12 , 2513, Henkelis J. J., Hardie M. J., Chem. Commun., 2015, 51 , 11929, Zarra S., Wood D. M., Roberts D. A., Nitschke J. R., Chem. Soc. Rev., 2015, 44 , 419, Cook T. R., Zheng Y.-R., Stang P. J., Chem. Rev., 2013, 113 , 734, Chakrabarty R., Mukherjee P. S., Stang P. J., Chem. Rev., 2011, 111 , 6810, Harris K., Fujita D., Fujita M., Chem. Commun., 2013, 49 , 6703, Jin P., Dalgarno S. J., Atwood J. L., Coord. Chem. Rev., 2010, 254 , 1760, Ward M. D., Chem. Commun., 2009. , 4487, Yoshizawa M., Klosterman J. K., Fujita M., Angew. Chem., Int. Ed., 2009, 48 , 3418, Fiedler D., Leung D. H., Bergman R. G., Raymond K. N., Acc. Chem. Res., 2005, 38 , 349 . - PubMed
-
- McConnell A. J., Wood C. S., Neelakandan P. P., Nitschke J. R. Chem. Rev. 2015;115:7729. - PubMed
-
- Han M., Michel R., He B., Chen Y.-S., Stalke D., John M., Clever G. H. Angew. Chem., Int. Ed. 2013;52:1319. - PubMed
-
- Han M., Luo Y., Damaschke B., Gómez L., Ribas X., Jose A., Peretzki P., Seibt M., Clever G. H. Angew. Chem., Int. Ed. 2016;55:445. - PubMed
-
- Bandara H. M. D., Burdette S. C. Chem. Soc. Rev. 2012;41:1809. - PubMed
LinkOut - more resources
Full Text Sources

