Redox-triggered cascade dearomative cyclizations enabled by hexafluoroisopropanol
- PMID: 30542574
- PMCID: PMC6240893
- DOI: 10.1039/c8sc03339k
Redox-triggered cascade dearomative cyclizations enabled by hexafluoroisopropanol
Abstract
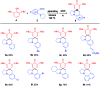
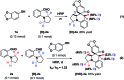
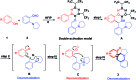
An unprecedented cascade dearomative cyclization through hydrogen-bonding-assisted hydride transfer is realized. The aggregate effect of HFIP enables the rapid buildup of polycyclic amines directly from phenols and o-aminobenzaldehydes via a cascade dearomatization/rearomatization/dearomatization sequence. This unique transformation addressed the drawbacks of hydride transfer-involved redox-neutral reactions.
Figures






References
-
- Campos K. R. Chem. Soc. Rev. 2007;36:1069. - PubMed
- Baudoin O. Chem. Soc. Rev. 2011;40:4902. - PubMed
- Shi L., Xia W. Chem. Soc. Rev. 2012;41:7687. - PubMed
- Mitchell E. A., Peschiulli A., Lefevre N., Meerpoel L., Maes B., Chem U. W. Chem.–Eur. J. 2012;18:10092. - PubMed
- Vo C. V. T., Bode J. W. J. Org. Chem. 2014;79:2809. - PubMed
- Seidel D. Acc. Chem. Res. 2015;48:317. - PMC - PubMed
- Mahato S., Jana C. K. Chem. Rec. 2016;16:1477. - PubMed
-
- Tobisu M., Chatani N. Angew. Chem., Int. Ed. 2006;45:1683. - PubMed
- Peng B., Maulide N. Chem.–Eur. J. 2013;19:13274. - PubMed
- Wang L., Xiao J. Adv. Synth. Catal. 2014;356:1137.
- Wang L., Xiao J. Top. Curr. Chem. 2016;374:17. - PubMed
- Wang L., Xiao J. Org. Chem. Front. 2016;3:635.
- Pan S. C. Beilstein J. Org. Chem. 2012;8:1374. - PMC - PubMed
- Haibach M. C., Seidel D. Angew. Chem., Int. Ed. 2014;53:5010. - PubMed
- Xiao M., Zhu S., Shen Y., Wang L., Xiao J. Chin. J. Org. Chem. 2018;38:328.
- Kwon S. J., Kim D. Y. Chem. Rec. 2016;16:1191. - PubMed
-
-
For examples of electrophilic alkenes, see:
- Pastine S. J., McQuaid K. M., Sames D. J. Am. Chem. Soc. 2005;127:12180. - PubMed
- Murarka S., Zhang C., Konieczynska M. D., Seidel D. Org. Lett. 2009;11:129. - PubMed
- Han Y., Han W., Hou X., Zhang X., Yuan W. Org. Lett. 2012;14:4054. - PubMed
- Yoshida T., Mori K. Chem. Commun. 2017;53:4319. - PubMed
- Ruble J. C., Hurd A. R., Johnson T. A., Sherry D. A., Barbachyn M. R., Toogood P. L., Bundy G. L., Graber D. R., Kamilar G. M. J. Am. Chem. Soc. 2009;131:3991. - PubMed
- Mori K., Ehara K., Kurihara K., Akiyama T. J. Am. Chem. Soc. 2011;133:6166. - PubMed
- Suh C. W., Kim D. Y. Org. Lett. 2014;16:5374. - PubMed
- Mori K., Kurihara K., Yabe S., Yamanaka M., Akiyama T. J. Am. Chem. Soc. 2014;136:3744. - PubMed
- Mori K., Isogai R., Kamei Y., Yamanaka M., Akiyama T. J. Am. Chem. Soc. 2018;140:6203. - PubMed
-
-
-
For representative examples, see:
- Jurberg I. D., Peng B., Woestefeld E., Wasserloos M., Maulide N. Angew. Chem., Int. Ed. 2012;51:1950. - PubMed
- Chen D., Han Z., He Y., Yu J., Gong L.-Z. Angew. Chem., Int. Ed. 2012;51:12307. - PubMed
- Vadola P. A., Sames D. J. Am. Chem. Soc. 2009;131:16525. - PMC - PubMed
- Barluenga J., Fananas-Mastral M., Aznar F., Valdes C. Angew. Chem., Int. Ed. 2008;47:6594. - PubMed
- Sugiishi T., Nakamura H. J. Am. Chem. Soc. 2012;134:2504. - PubMed
- Theunissen C., Metayer B., Henry N., Compain G., Marrot J., Martin-Mingot A., Thibaudeau S., Evano G. J. Am. Chem. Soc. 2014;136:12528. - PubMed
- Cui L., Peng Y., Zhang L. M. J. Am. Chem. Soc. 2009;131:8394. - PubMed
- Kang Y. K., Kim S. M., Kim D. Y. J. Am. Chem. Soc. 2012;132:11847. - PubMed
-
-
-
For recently developed hydride acceptors, see:
- Chang Y.-Z., Li M.-L., Zhao W.-F., Wen X., Sun H., Xu Q.-L. J. Org. Chem. 2015;80:9620. - PubMed
- Suh C. W., Kwon S. J., Kim D. Y. Org. Lett. 2017;19:1334. - PubMed
- Liu S., Qu J., Wang B. Chem. Commun. 2018;54:7928. - PubMed
- Li S.-S., Zhou L., Wang L., Zhao H., Yu L., Xiao J. Org. Lett. 2018;20:138. - PubMed
- Idiris F. I. M., Majesté C. E., Craven G. B., Jones C. R., Chem. Sci., 2018, 9 , 2873 , ; For an intermolecular hydride transfer, see: . - PMC - PubMed
- Chen W., Ma L., Paul A., Seidel D. Nat. Chem. 2018;10:165. - PMC - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

