Facile folding of insulin variants bearing a prosthetic C-peptide prepared by α-ketoacid-hydroxylamine (KAHA) ligation
- PMID: 30542587
- PMCID: PMC6243641
- DOI: 10.1039/c8sc03738h
Facile folding of insulin variants bearing a prosthetic C-peptide prepared by α-ketoacid-hydroxylamine (KAHA) ligation
Abstract
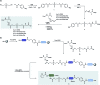
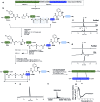
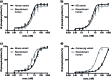
The chemical synthesis of insulin is an enduring challenge due to the hydrophobic peptide chains and construction of the correct intermolecular disulfide pattern. We report a new approach to the chemical synthesis of insulin using a short, traceless, prosthetic C-peptide that facilitates the formation of the correct disulfide pattern during folding and its removal by basic treatment. The linear precursor is assembled by an ester forming α-ketoacid-hydroxylamine (KAHA) ligation that provides access to the linear insulin precursors in good yield from two readily prepared segments. This convergent and flexible route provides access to various human, mouse, and guinea pig insulins containing a single homoserine mutation that shows no detrimental effect on the biological activities.
Figures


Similar articles
-
Chemical Protein Synthesis with the α-Ketoacid-Hydroxylamine Ligation.Acc Chem Res. 2017 Sep 19;50(9):2104-2115. doi: 10.1021/acs.accounts.7b00277. Epub 2017 Aug 29. Acc Chem Res. 2017. PMID: 28849903
-
Aspartic Acid Forming α-Ketoacid-Hydroxylamine (KAHA) Ligations with (S)-4,4-Difluoro-5-oxaproline.J Org Chem. 2020 Feb 7;85(3):1352-1364. doi: 10.1021/acs.joc.9b02271. Epub 2020 Jan 7. J Org Chem. 2020. PMID: 31840512
-
Chemical Synthesis of the 20 kDa Heme Protein Nitrophorin 4 by α-Ketoacid-Hydroxylamine (KAHA) Ligation.Angew Chem Int Ed Engl. 2015 Oct 26;54(44):12996-3001. doi: 10.1002/anie.201505379. Epub 2015 Sep 8. Angew Chem Int Ed Engl. 2015. PMID: 26346606
-
The Chemical Synthesis of Insulin: An Enduring Challenge.Chem Rev. 2021 Apr 28;121(8):4531-4560. doi: 10.1021/acs.chemrev.0c01251. Epub 2021 Mar 9. Chem Rev. 2021. PMID: 33689304 Review.
-
Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation.Amino Acids. 2018 Jan;50(1):39-68. doi: 10.1007/s00726-017-2516-0. Epub 2017 Nov 28. Amino Acids. 2018. PMID: 29185032 Review.
Cited by
-
A Versatile "Synthesis Tag" (SynTag) for the Chemical Synthesis of Aggregating Peptides and Proteins.J Am Chem Soc. 2024 Dec 18;146(50):34887-34899. doi: 10.1021/jacs.4c14247. Epub 2024 Dec 5. J Am Chem Soc. 2024. PMID: 39639492 Free PMC article.
-
Diselenide-bond replacement of the external disulfide bond of insulin increases its oligomerization leading to sustained activity.Commun Chem. 2023 Nov 21;6(1):258. doi: 10.1038/s42004-023-01056-4. Commun Chem. 2023. PMID: 37989850 Free PMC article.
-
Chemical synthesis of Torenia plant pollen tube attractant proteins by KAHA ligation.RSC Chem Biol. 2022 Mar 18;3(6):721-727. doi: 10.1039/d2cb00039c. eCollection 2022 Jun 8. RSC Chem Biol. 2022. PMID: 35755195 Free PMC article.
-
Synthesis and Characterization of an A6-A11 Methylene Thioacetal Human Insulin Analogue with Enhanced Stability.J Med Chem. 2019 Dec 26;62(24):11437-11443. doi: 10.1021/acs.jmedchem.9b01589. Epub 2019 Dec 13. J Med Chem. 2019. PMID: 31804076 Free PMC article.
-
Chemical synthesis of the EPF-family of plant cysteine-rich proteins and late-stage dye attachment by chemoselective amide-forming ligations.RSC Chem Biol. 2022 Oct 19;3(12):1422-1431. doi: 10.1039/d2cb00155a. eCollection 2022 Nov 30. RSC Chem Biol. 2022. PMID: 36544577 Free PMC article.
References
-
- Mayer J. P., Zhang F., DiMarchi R. D. Biopolymers. 2007;88:687–713. - PubMed
-
- Katsoyannis P. G. Diabetes. 1964;13:339–348. - PubMed
-
- Katsoyannis P. G., Traketellis A. C., Zalut C., Johnson S., Tometsko A., Schwartz G., Ginos J. Biochemistry. 1967;9:2658–2668. - PubMed
-
- Liu F., Luo E. Y., Flora D. B., Mayer J. P. Org. Lett. 2013;15:960–963. - PubMed
-
- Liu F., Luo E. Y., Flora D. B., Mezo A. R. Angew. Chem., Int. Ed. 2014;53:3983–3987. - PubMed
LinkOut - more resources
Full Text Sources

