Regio- and chemoselective Csp3-H arylation of benzylamines by single electron transfer/hydrogen atom transfer synergistic catalysis
- PMID: 30542595
- PMCID: PMC6244453
- DOI: 10.1039/c8sc02965b
Regio- and chemoselective Csp3-H arylation of benzylamines by single electron transfer/hydrogen atom transfer synergistic catalysis
Abstract
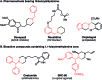
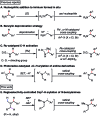
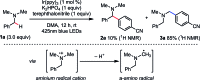
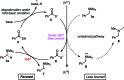
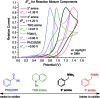
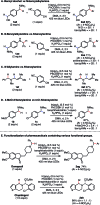
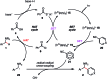
We present a highly regio- and chemoselective Csp3-H arylation of benzylamines mediated by synergy of single electron transfer (SET) and hydrogen atom transfer (HAT) catalysis. Under well precedented SET catalysis alone, the arylation reaction of N,N-dimethylbenzylamine proceeded via aminium radical cation formation and selectively targeted the N-methyl group. In contrast, addition of PhC(O)SH as a HAT catalyst precursor completely switched the regioselectivity to Csp3-H arylation at the N-benzylic position. Measurement of oxidation potentials indicated that the conjugate base of PhC(O)SH is oxidized in preference to the substrate amine. The discovery of the thiocarboxylate as a novel HAT catalyst allowed for the selective generation of the sulfur-centered radical, so that the N-benzyl selectivity was achieved by overriding the inherent N-methyl and/or N-methylene selectivity under SET catalysis conditions. While visible light-driven α-C-H functionalization of amines has mostly been demonstrated with aniline derivatives and tetrahydroisoquinolines (THIQs), our method is applicable to a variety of primary, secondary and tertiary benzylamines for efficient N-benzylic C-H arylation. Functional group tolerance was high, and various 1,1-diarylmethylamines, including an α,α,α-trisubstituted amine, were obtained in good to excellent yield (up to 98%). Importantly, the reaction is applicable to late-stage functionalization of pharmaceuticals.
Figures
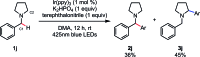
Similar articles
-
Site-selective α-C(sp3)-H arylation of dialkylamines via hydrogen atom transfer catalysis-enabled radical aryl migration.Nat Commun. 2024 Aug 9;15(1):6791. doi: 10.1038/s41467-024-51239-3. Nat Commun. 2024. PMID: 39117735 Free PMC article.
-
Triarylamine-based porous coordination polymers performing both hydrogen atom transfer and photoredox catalysis for regioselective α-amino C(sp3)-H arylation.Chem Sci. 2021 May 18;12(24):8512-8520. doi: 10.1039/d1sc00828e. Chem Sci. 2021. PMID: 34221332 Free PMC article.
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
C-H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals.Chem Sci. 2020 Nov 16;11(48):12974-12993. doi: 10.1039/d0sc04881j. Chem Sci. 2020. PMID: 34123240 Free PMC article. Review.
-
From α-arylation of olefins to acylation with aldehydes: a journey in regiocontrol of the Heck reaction.Acc Chem Res. 2011 Aug 16;44(8):614-26. doi: 10.1021/ar200053d. Epub 2011 May 25. Acc Chem Res. 2011. PMID: 21612205 Review.
Cited by
-
Photocatalytic C(sp3) radical generation via C-H, C-C, and C-X bond cleavage.Chem Sci. 2022 Apr 18;13(19):5465-5504. doi: 10.1039/d2sc00202g. eCollection 2022 May 18. Chem Sci. 2022. PMID: 35694342 Free PMC article. Review.
-
Benzhydryl Amines: Synthesis and Their Biological Perspective.ACS Omega. 2019 Dec 17;5(1):19-30. doi: 10.1021/acsomega.9b03090. eCollection 2020 Jan 14. ACS Omega. 2019. PMID: 31956747 Free PMC article. Review.
-
Aliphatic C-H Functionalization Using Pyridine N-Oxides as H-Atom Abstraction Agents.ACS Catal. 2022 Aug 19;12(16):10499-10505. doi: 10.1021/acscatal.2c02997. Epub 2022 Aug 10. ACS Catal. 2022. PMID: 37727583 Free PMC article.
-
Photocatalytic Hydroaminoalkylation of Styrenes with Unprotected Primary Alkylamines.J Am Chem Soc. 2021 Oct 6;143(39):15936-15945. doi: 10.1021/jacs.1c07401. Epub 2021 Sep 20. J Am Chem Soc. 2021. PMID: 34543004 Free PMC article.
-
Polarity-Driven Thiyl Radical-Catalyzed Aerobic Debenzylation of Ethers and Amines.J Org Chem. 2024 Oct 18;89(20):15062-15067. doi: 10.1021/acs.joc.4c01796. Epub 2024 Oct 9. J Org Chem. 2024. PMID: 39380545 Free PMC article.
References
-
- Labinger J. A., Bercaw J. E. Nature. 2002;417:507–514. - PubMed
- Godula K., Sames D. Science. 2006;312:67–72. - PubMed
- Bergman R. G. Nature. 2007;446:391–393. - PubMed
- Campos K. R. Chem. Soc. Rev. 2007;36:1069–1084. - PubMed
- Newhuse T., Baran P. S. Angew. Chem., Int. Ed. 2011;50:3362–3374. - PMC - PubMed
- McMurray L., O'Hara F., Gaunt M. J. Chem. Soc. Rev. 2011;40:1885–1898. - PubMed
- White M. C. Science. 2012;335:807–809. - PubMed
- Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960–9009. - PubMed
- Wencel-Delord J., Glorius F. Nat. Chem. 2013;5:369–375. - PubMed
- Cernak T., Dykstra K. D., Tyagarajan S., Vachal P., Krska S. W. Chem. Soc. Rev. 2016;45:546–574. - PubMed
- Chu J. C. K., Rovis T. Angew. Chem., Int. Ed. 2018;57:62–101. - PMC - PubMed
-
- Vitaku E., Smith D. T., Njardarson J. T. J. Med. Chem. 2014;57:10257–10274. - PubMed
-
- Ameen D., Snape T. J. Med. Chem. Commun. 2013;4:893–907.
-
-
; during the preparation of this manuscript, Doyle and co-workers reported an elegant Ni-catalyzed three-component coupling reaction, see:
- Böhme H., Plappert P. Chem. Ber. 1975;108:2827–2833.
- Murai T., Asai F. J. Am. Chem. Soc. 2007;129:780–781. - PubMed
- He S., Xiao J., Dulcey A. E., Lin B., Rolt A., Hu Z., Hu X., Wang A. Q., Xu X., Southall N., Ferrer M., Zheng W., Liang T. J., Marugan J. J. J. Med. Chem. 2016;59:841–853. - PMC - PubMed
- Xie L.-G., Dixon D. J. Chem. Sci. 2017;8:7492–7497. - PMC - PubMed
- Heinz C., Lutz J. P., Simmons E. M., Miller M. M., Ewing W. R., Doyle A. G. J. Am. Chem. Soc. 2018;140:2292–2300. - PMC - PubMed
-
-
-
For selected examples of N-benzylic C–H arylation via addition of aryl nucleophiles to iminium ions, see:
- Li Z., Li C.-J. J. Am. Chem. Soc. 2005;127:6968–6969. - PubMed
- Li Z., Bohle D. S., Li C.-J. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8928–8933. - PMC - PubMed
- Muramatsu W., Nakano K., Li C.-J. Org. Lett. 2013;15:3650–3653. - PubMed
-
LinkOut - more resources
Full Text Sources

