PI3Kβ inhibitor AZD6482 exerts antiproliferative activity and induces apoptosis in human glioblastoma cells
- PMID: 30542720
- PMCID: PMC6278584
- DOI: 10.3892/or.2018.6845
PI3Kβ inhibitor AZD6482 exerts antiproliferative activity and induces apoptosis in human glioblastoma cells
Abstract
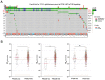
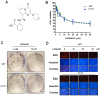
Glioblastoma is the most common type of primary brain tumour in adults, and its pathogenesis is particularly complicated. Among the many possible mechanisms underlying its pathogenesis, hyperactivation of the PI3K/Akt pathway is essential to the occurrence and development of glioma through the loss of PTEN or somatic activating mutations in PIK3CA. In the present study, we investigated the effect of the PI3Kβ inhibitor AZD6482 on glioma cells. The CCK-8 assay showed dose-dependent cytotoxicity in glioma cell lines treated with AZD6482. Additionally, AZD6482 treatment was found to significantly induce apoptosis and cell cycle arrest as detected using flow cytometry. Moreover, as shown using western blot analysis, the levels of p-AKT, p-GSK-3β, Bcl-2, and cyclin D1 were decreased after AZD6482 treatment. In addition, we found that AZD6482 inhibited the migration and invasion of glioma cells as detected by wound healing and Transwell invasion assays. Taken together, our findings indicate that AZD6482 exerts an antitumour effect by inhibiting proliferation and inducing apoptosis in human glioma cells. AZD6482 may be applied as an adjuvant therapy to improve the therapeutic efficacy of glioblastoma treatment.
Figures



References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

