Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial
- PMID: 30543347
- PMCID: PMC6439561
- DOI: 10.1001/jamaoncol.2018.4475
Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial
Abstract
Importance: The phosphatidylinositol 3-kinase (PI3K) pathway is frequently activated in patients with estrogen receptor-positive (ER+), endocrine therapy-resistant breast cancers.
Objective: To assess the maximum tolerated dose (MTD), safety, and activity of alpelisib, an oral, PI3Kα-specific inhibitor, plus fulvestrant in patients with ER+ advanced breast cancer (ABC).
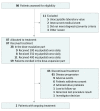
Design, setting, and participants: An open-label, single-arm, phase 1b study of alpelisib plus fulvestrant was conducted at 10 centers in 5 countries. Participants were 87 postmenopausal women with PIK3CA-altered or PIK3CA-wild-type ER+ ABC, whose cancer progressed during or after antiestrogen therapy. The study began enrolling patients October 5, 2010, and the data cutoff was March 22, 2017.
Interventions: Escalating doses of alpelisib were administered once daily, starting at 300 mg, plus fixed-dose fulvestrant, 500 mg, in the dose-escalation phase; alpelisib at the recommended phase 2 dose plus fulvestrant in the dose-expansion phase.
Main outcomes and measures: The primary end point was determination of the MTD of once-daily alpelisib plus fulvestrant. Secondary end points included safety and preliminary activity.
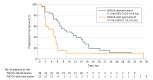
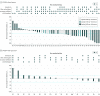
Results: From October 5, 2010, to March 22, 2017, 87 women (median age: 58 years [range, 37-79 years]; median of 5 prior lines of antineoplastic therapy) received escalating once-daily doses of alpelisib (300 mg, n = 9; 350 mg, n = 8; 400 mg, n = 70) plus fixed-dose fulvestrant (500 mg). During dose escalation, dose-limiting toxic effects were reported in 1 patient (alpelisib, 400 mg): diarrhea (grade 2), vomiting, fatigue, and decreased appetite (all grade 3). The MTD of alpelisib when combined with fulvestrant was 400 mg once daily, and the recommended phase 2 dose was 300 mg once daily. Overall, the most frequent grade 3/4 adverse events with alpelisib, 400 mg, once daily (≥10% of patients), regardless of causality, were hyperglycemia (19 [22%]) and maculopapular rash (11 [13%]); 9 patients permanently discontinued therapy owing to adverse events. Median progression-free survival at the MTD was 5.4 months (95% CI, 4.6-9.0 months). Median progression-free survival with alpelisib, 300 to 400 mg, once daily plus fulvestrant was longer in patients with PIK3CA-altered tumors (9.1 months; 95% CI, 6.6-14.6 months) vs wild-type tumors (4.7 months; 95% CI, 1.9-5.6 months). Overall response rate in the PIK3CA-altered group was 29% (95% CI, 17%-43%), with no objective tumor responses in the wild-type group.
Conclusions and relevance: Alpelisib plus fulvestrant has a manageable safety profile in patients with ER+ ABC, and data suggest that this combination may have greater clinical activity in PIK3CA-altered vs wild-type tumors.
Trial registration: ClinicalTrials.gov identifier: NCT01219699.
Conflict of interest statement
Figures
References
-
- Fitzmaurice C, Allen C, Barber RM, et al. ; Global Burden of Disease Cancer Collaboration . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):-. doi: 10.1001/jamaoncol.2016.5688 - DOI - PMC - PubMed
-
- Shah PD, Dickler MN. Endocrine therapy for advanced breast cancer. Clin Adv Hematol Oncol. 2014;12(4):214-223. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer. Version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 12, 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

