Cost-effectiveness and Budgetary Consequence Analysis of Durvalumab Consolidation Therapy vs No Consolidation Therapy After Chemoradiotherapy in Stage III Non-Small Cell Lung Cancer in the Context of the US Health Care System
- PMID: 30543349
- PMCID: PMC6439842
- DOI: 10.1001/jamaoncol.2018.5449
Cost-effectiveness and Budgetary Consequence Analysis of Durvalumab Consolidation Therapy vs No Consolidation Therapy After Chemoradiotherapy in Stage III Non-Small Cell Lung Cancer in the Context of the US Health Care System
Abstract
Importance: In early 2018, durvalumab became the first immunotherapy to be approved for adjuvant treatment of patients with unresectable stage III non-small cell lung cancer (NSCLC) whose cancer has not progressed after definitive chemoradiotherapy. However, the cost-effectiveness and potential economic implications of using this high-priced therapy in this indication are unknown to date.
Objective: To explore the cost-effectiveness and potential budgetary consequences of durvalumab consolidation therapy vs no consolidation therapy after chemoradiotherapy in stage III NSCLC in the context of the US health care system.
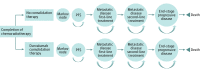
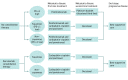
Design, setting, and participants: A decision analytic microsimulation model was developed in an academic medical setting to compare the following 2 postchemoradiotherapy strategies: all patients receive no consolidation therapy until progression vs all patients receive durvalumab consolidation therapy until progression or for a maximum of 1 year. The potential budgetary consequence was calculated by applying the proportion of patients with NSCLC who were diagnosed in stage III and received chemoradiotherapy to the projected number of annual new cases for 2018 to 2022 to find total eligible patients and then multiplied by the mean difference in annual cost between the strategies over this 5-year period. Simulated conditions were matched to those of the PACIFIC phase 3 randomized clinical trial and reasonable treatment strategies for metastatic NSCLC. All simulated patients begin disease free after having received radical treatment with chemoradiotherapy and are followed up as they progress to metastatic disease first-line treatment, metastatic disease second-line treatment, end-stage progressive disease, and death.
Main outcomes and measures: The main outcome of this study was the incremental cost-effectiveness ratio of durvalumab consolidation therapy vs no consolidation therapy, given as aggregate cost of treatment per quality-adjusted life-year gained.
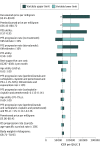
Results: Among 2 million simulated patients, durvalumab consolidation therapy was cost-effective compared with no consolidation therapy at a $100 000 per quality-adjusted life-year willingness-to-pay threshold, with an estimated incremental cost-effectiveness ratio of $67 421 per quality-adjusted life-year, and would contribute an additional $768 million to national cancer spending in year 1. The annual budgetary consequence would then decrease to $241 million in year 5.
Conclusions and relevance: Durvalumab consolidation therapy represents an indication where expensive immunotherapies can be cost-effective. Treating with immunotherapy earlier in the course of cancer progression can provide significant value, despite having a substantial budgetary consequence.
Conflict of interest statement
Figures
Comment in
-
Changes to Model Assumptions of the Cost-effectiveness of Durvalumab Therapy for Non-Small Cell Lung Cancer-In Reply.JAMA Oncol. 2019 Jul 1;5(7):1066-1067. doi: 10.1001/jamaoncol.2019.1100. JAMA Oncol. 2019. PMID: 31095247 No abstract available.
-
Changes to Model Assumptions of the Cost-effectiveness of Durvalumab Therapy for Non-Small Cell Lung Cancer.JAMA Oncol. 2019 Jul 1;5(7):1066. doi: 10.1001/jamaoncol.2019.1088. JAMA Oncol. 2019. PMID: 31095252 No abstract available.
Similar articles
-
Cost effectiveness of immune checkpoint inhibitors for treatment of non-small cell lung cancer: A systematic review.PLoS One. 2020 Sep 2;15(9):e0238536. doi: 10.1371/journal.pone.0238536. eCollection 2020. PLoS One. 2020. PMID: 32877435 Free PMC article.
-
Cost-effectiveness and Net Monetary Benefit of Durvalumab Consolidation Therapy Versus No Consolidation Therapy After Chemoradiotherapy in Stage III Non-small Cell Lung Cancer in the Italian National Health Service.Clin Ther. 2020 May;42(5):830-847. doi: 10.1016/j.clinthera.2020.03.012. Epub 2020 Apr 27. Clin Ther. 2020. PMID: 32354495
-
Budget impact analysis of durvalumab consolidation therapy vs no consolidation therapy after chemoradiotherapy in stage III non-small cell lung cancer in the context of the Chilean health care system.PLoS One. 2024 Jul 26;19(7):e0307473. doi: 10.1371/journal.pone.0307473. eCollection 2024. PLoS One. 2024. PMID: 39058755 Free PMC article.
-
International Cost-Effectiveness Analysis of Durvalumab in Stage III Non-Small Cell Lung Cancer.JAMA Netw Open. 2024 May 1;7(5):e2413938. doi: 10.1001/jamanetworkopen.2024.13938. JAMA Netw Open. 2024. PMID: 38814640 Free PMC article.
-
Immune checkpoint-inhibitors and chemoradiation in stage III unresectable non-small cell lung cancer.Lung Cancer. 2019 Aug;134:259-267. doi: 10.1016/j.lungcan.2019.05.027. Epub 2019 May 29. Lung Cancer. 2019. PMID: 31319991 Review.
Cited by
-
Cost-effectiveness of pembrolizumab for advanced non-small cell lung cancer patients with varying comorbidity burden.PLoS One. 2020 Jan 29;15(1):e0228288. doi: 10.1371/journal.pone.0228288. eCollection 2020. PLoS One. 2020. PMID: 31995619 Free PMC article.
-
Cost-effectiveness analysis of durvalumab plus chemotherapy as first-line treatment for biliary tract cancer.Front Public Health. 2023 Feb 10;11:1046424. doi: 10.3389/fpubh.2023.1046424. eCollection 2023. Front Public Health. 2023. PMID: 36844853 Free PMC article.
-
Cost-effectiveness of neoadjuvant pembrolizumab plus chemotherapy with adjuvant pembrolizumab for early-stage non-small cell lung cancer in the United States.Front Immunol. 2023 Sep 26;14:1268070. doi: 10.3389/fimmu.2023.1268070. eCollection 2023. Front Immunol. 2023. PMID: 37822936 Free PMC article.
-
Cost effectiveness of immune checkpoint inhibitors for treatment of non-small cell lung cancer: A systematic review.PLoS One. 2020 Sep 2;15(9):e0238536. doi: 10.1371/journal.pone.0238536. eCollection 2020. PLoS One. 2020. PMID: 32877435 Free PMC article.
-
Cost-effectiveness of first-line immunotherapies for advanced non-small cell lung cancer.Cancer Med. 2023 Apr;12(7):8838-8850. doi: 10.1002/cam4.5632. Epub 2023 Jan 18. Cancer Med. 2023. PMID: 36653947 Free PMC article.
References
-
- American Cancer Society Cancer Facts & Figures 2018. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts.... Accessed March 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

