Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial
- PMID: 30543350
- PMCID: PMC6439568
- DOI: 10.1001/jamaoncol.2018.5543
Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial
Erratum in
-
Error in Author Affiliation.JAMA Oncol. 2019 Feb 1;5(2):271. doi: 10.1001/jamaoncol.2019.0117. JAMA Oncol. 2019. PMID: 30763426 Free PMC article. No abstract available.
Abstract
Importance: In clinical practice, patients with primary metastatic renal cell carcinoma (mRCC) have been offered cytoreductive nephrectomy (CN) followed by targeted therapy, but the optimal sequence of surgery and systemic therapy is unknown.
Objective: To examine whether a period of sunitinib therapy before CN improves outcome compared with immediate CN followed by sunitinib.
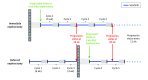
Design, setting, and participants: This randomized clinical trial began as a phase 3 trial on July 14, 2010, and continued until March 24, 2016, with a median follow-up of 3.3 years and a clinical cutoff date for this report of May 5, 2017. Patients with mRCC of clear cell subtype, resectable primary tumor, and 3 or fewer surgical risk factors were studied.
Interventions: Immediate CN followed by sunitinib therapy vs treatment with 3 cycles of sunitinib followed by CN in the absence of progression followed by sunitinib therapy.
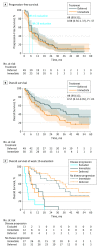
Main outcomes and measures: Progression-free survival was the primary end point, which needed a sample size of 458 patients. Because of poor accrual, the independent data monitoring committee endorsed reporting the intention-to-treat 28-week progression-free rate (PFR) instead. Overall survival (OS), adverse events, and postoperative progression were secondary end points.
Results: The study closed after 5.7 years with 99 patients (80 men and 19 women; mean [SD] age, 60 [8.5] years). The 28-week PFR was 42% in the immediate CN arm (n = 50) and 43% in the deferred CN arm (n = 49) (P = .61). The intention-to-treat OS hazard ratio of deferred vs immediate CN was 0.57 (95% CI, 0.34-0.95; P = .03), with a median OS of 32.4 months (95% CI, 14.5-65.3 months) in the deferred CN arm and 15.0 months (95% CI, 9.3-29.5 months) in the immediate CN arm. In the deferred CN arm, 48 of 49 patients (98%; 95% CI, 89%-100%) received sunitinib vs 40 of 50 (80%; 95% CI, 67%-89%) in the immediate arm. Systemic progression before planned CN in the deferred CN arm resulted in a per-protocol recommendation against nephrectomy in 14 patients (29%; 95% CI, 18%-43%).
Conclusions and relevance: Deferred CN did not improve the 28-week PFR. With the deferred approach, more patients received sunitinib and OS results were higher. Pretreatment with sunitinib may identify patients with inherent resistance to systemic therapy before planned CN. This evidence complements recent data from randomized clinical trials to inform treatment decisions in patients with primary clear cell mRCC requiring sunitinib.
Trial registration: ClinicalTrials.gov identifier: NCT01099423.
Conflict of interest statement
Figures
Comment in
-
Cytoreductive Nephrectomy in Metastatic Renal Cell Cancer: Not All That It's Cut Out to Be.JAMA Oncol. 2019 Feb 1;5(2):171-172. doi: 10.1001/jamaoncol.2018.5503. JAMA Oncol. 2019. PMID: 30543365 No abstract available.
-
Editorial Comment: Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial.Int Braz J Urol. 2020 May-Jun;46(3):476. doi: 10.1590/S1677-5538.IBJU.2020.03.12. Int Braz J Urol. 2020. PMID: 32167723 Free PMC article. No abstract available.
-
Re: Comparison of immediate vs. deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial.Transl Cancer Res. 2019 Mar;8(Suppl 2):S208-S210. doi: 10.21037/tcr.2019.03.08. Transl Cancer Res. 2019. PMID: 35117099 Free PMC article. No abstract available.
References
-
- Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R; European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group . Radical nephrectomy plus interferon-alfa–based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966-970. doi: 10.1016/S0140-6736(01)06103-7 - DOI - PubMed
-
- Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171(3):1071-1076. doi: 10.1097/01.ju.0000110610.61545.ae - DOI - PubMed

