Cysteine-Directed Bioconjugation of a Platinum(II)-Acridine Anticancer Agent
- PMID: 30543413
- PMCID: PMC12184284
- DOI: 10.1021/acs.inorgchem.8b02717
Cysteine-Directed Bioconjugation of a Platinum(II)-Acridine Anticancer Agent
Abstract
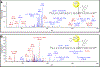
Classical maleimide Michael addition chemistry in conjunction with copper-free click chemistry was investigated as a synthetic strategy to attach cytotoxic platinum-acridine hybrid agents to carrier proteins. The structural integrity and selectivity of the model payloads, which were validated in human serum albumin (HSA) using mass spectrometric analysis and heteronuclear 2D 1H-15N HSQC NMR experiments, may have broad utility for the targeted delivery of highly cytotoxic platinum acridines and other nonclassical platinum containing anticancer agents.
Conflict of interest statement
Notes
The authors declare no competing financial interests.
Figures



References
-
- Barenholz Y Doxil®-the first FDA-approved nano-drug: lessons learned. J. Control. Release 2012, 160, 117–134. - PubMed
-
- Ding S; Bierbach U Target-selective delivery and activation of platinum-based anticancer agents. Future Med. Chem. 2015, 7, 911–927. - PubMed
-
- Park K Albumin: a versatile carrier for drug delivery. J. Control. Release 2012, 157, 3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

