Liver repair and regeneration after ischemia-reperfusion injury is associated with prolonged fibrosis
- PMID: 30543462
- PMCID: PMC6459287
- DOI: 10.1152/ajpgi.00154.2018
Liver repair and regeneration after ischemia-reperfusion injury is associated with prolonged fibrosis
Abstract
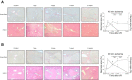
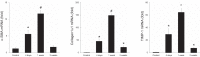
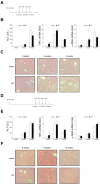
Liver recovery after hepatic ischemia-reperfusion (I/R) injury is characterized by clearance of dead tissue and its replacement with functional liver parenchyma. Previous reports have observed fibrosis after liver I/R. To determine whether liver fibrosis after I/R was a pathologic consequence of the injury response, we assessed the development of liver fibrosis after I/R and its impact on subsequent insult. A murine model of partial I/R was used to induce liver injury and study the reparative response. During liver remodeling after I/R, expression of the profibrotic genes increased in the ischemic liver. Histologically, α-smooth muscle actin (α-SMA)-positive hepatic stellate cells (HSCs)/myofibroblasts increased, and collagen deposition was enhanced along the injured site. Selective staining experiments showed that HSCs, not portal fibroblasts, were the major source of myofibroblasts. During liver repair after I/R, liver fibrosis was readily observed at the interface between necrotic tissue and regenerating liver in association with HSCs/myofibroblasts. The number of HSCs/myofibroblasts decreasing shortly after the full resolution of necrotic injury and restoration are normal liver architecture. However, liver fibrosis persisted for several more weeks before gradually resolving. Resolution of liver fibrosis was accompanied by upregulated expression of matrix metalloproteinase-13. After resolution of fibrosis, the administration of CCl4 did not result in exacerbated liver injury, suggesting that I/R injury does not predispose the liver to future fibrotic insults. The data suggest that liver fibrosis is a component of tissue repair after I/R, is caused by myofibroblasts derived from HSC, and does not increase susceptibility of the liver to subsequent hepatic injury. NEW & NOTEWORTHY This study is the first to assess pathology of liver fibrosis during the reparative process after ischemia-reperfusion (I/R) injury. Here we show that profibrotic gene expression increased in the liver after I/R, and collagen accumulation produced by hepatic stellate cells (HSCs)/myofibroblasts enhanced at the interface between necrotic tissue and regenerating liver. Liver fibrosis gradually resolved concomitant with decreasing activation of HSC and upregulating matrix metalloproteinase-13. After resolution of fibrosis, the liver was not more susceptible to subsequent hepatic injury.
Keywords: hepatic stellate cells; liver fibrosis; liver regeneration; liver repair.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Fibrotic liver has prompt recovery after ischemia-reperfusion injury.Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G390-G400. doi: 10.1152/ajpgi.00137.2019. Epub 2020 Jan 21. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31961717 Free PMC article.
-
Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury.Am J Physiol Gastrointest Liver Physiol. 2018 Apr 1;314(4):G471-G482. doi: 10.1152/ajpgi.00153.2017. Epub 2018 Jan 11. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29351389 Free PMC article.
-
Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice.J Hepatol. 2019 Sep;71(3):573-585. doi: 10.1016/j.jhep.2019.04.012. Epub 2019 May 7. J Hepatol. 2019. PMID: 31071368
-
Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells.Int J Mol Sci. 2024 Jul 18;25(14):7873. doi: 10.3390/ijms25147873. Int J Mol Sci. 2024. PMID: 39063116 Free PMC article. Review.
-
Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets.Hepatobiliary Pancreat Dis Int. 2018 Jun;17(3):192-197. doi: 10.1016/j.hbpd.2018.04.003. Epub 2018 Apr 21. Hepatobiliary Pancreat Dis Int. 2018. PMID: 29709350 Review.
Cited by
-
TAK242 suppresses the TLR4 signaling pathway and ameliorates DCD liver IRI in rats.Mol Med Rep. 2019 Sep;20(3):2101-2110. doi: 10.3892/mmr.2019.10439. Epub 2019 Jun 27. Mol Med Rep. 2019. PMID: 31257518 Free PMC article.
-
Propofol Pretreatment Inhibits Liver Damage in Mice with Hepatic Ischemia/Reperfusion Injury and Protects Human Hepatocyte in Hypoxia/Reoxygenation.Turk J Gastroenterol. 2023 Nov;34(11):1171-1179. doi: 10.5152/tjg.2023.21218. Turk J Gastroenterol. 2023. PMID: 37768306 Free PMC article.
-
Molecular Mechanisms of Ischaemia-Reperfusion Injury and Regeneration in the Liver-Shock and Surgery-Associated Changes.Int J Mol Sci. 2022 Oct 26;23(21):12942. doi: 10.3390/ijms232112942. Int J Mol Sci. 2022. PMID: 36361725 Free PMC article. Review.
-
New Insights in Mechanisms and Therapeutics for Short- and Long-Term Impacts of Hepatic Ischemia Reperfusion Injury Post Liver Transplantation.Int J Mol Sci. 2021 Jul 30;22(15):8210. doi: 10.3390/ijms22158210. Int J Mol Sci. 2021. PMID: 34360975 Free PMC article. Review.
-
Fibrotic liver has prompt recovery after ischemia-reperfusion injury.Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G390-G400. doi: 10.1152/ajpgi.00137.2019. Epub 2020 Jan 21. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31961717 Free PMC article.
References
-
- Amer AO, Probert PM, Dunn M, Knight M, Vallance AE, Flecknell PA, Oakley F, Cameron I, White SA, Blain PG, Wright MC. Sustained isoprostane E2 elevation, inflammation and fibrosis after acute ischaemia-reperfusion injury are reduced by pregnane X receptor activation. PLoS One 10: e0136173, 2015. doi:10.1371/journal.pone.0136173. - DOI - PMC - PubMed
-
- Dechêne A, Sowa JP, Gieseler RK, Jochum C, Bechmann LP, El Fouly A, Schlattjan M, Saner F, Baba HA, Paul A, Dries V, Odenthal M, Gerken G, Friedman SL, Canbay A. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology 52: 1008–1016, 2010. doi:10.1002/hep.23754. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

