Structure, heterogeneity and developability assessment of therapeutic antibodies
- PMID: 30543482
- PMCID: PMC6380400
- DOI: 10.1080/19420862.2018.1553476
Structure, heterogeneity and developability assessment of therapeutic antibodies
Abstract
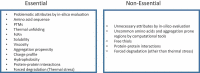
Increasing attention has been paid to developability assessment with the understanding that thorough evaluation of monoclonal antibody lead candidates at an early stage can avoid delays during late-stage development. The concept of developability is based on the knowledge gained from the successful development of approximately 80 marketed antibody and Fc-fusion protein drug products and from the lessons learned from many failed development programs over the last three decades. Here, we reviewed antibody quality attributes that are critical to development and traditional and state-of-the-art analytical methods to monitor those attributes. Based on our collective experiences, a practical workflow is proposed as a best practice for developability assessment including in silico evaluation, extended characterization and forced degradation using appropriate analytical methods that allow characterization with limited material consumption and fast turnaround time.
Keywords: Developability; monoclonal antibody; posttranslational modifications.
Figures
References
-
- Dobson CL, Devine PW, Phillips JJ, Higazi DR, Lloyd C, Popovic B, Arnold J, Buchanan A, Lewis A, Goodman J, et al. Engineering the surface properties of a human monoclonal antibody prevents self-association and rapid clearance in vivo. Sci Rep. 2016;6:38644. doi:10.1038/srep38644. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources