Sensitivity to inhibition of DNA repair by Olaparib in novel oropharyngeal cancer cell lines infected with Human Papillomavirus
- PMID: 30543656
- PMCID: PMC6292594
- DOI: 10.1371/journal.pone.0207934
Sensitivity to inhibition of DNA repair by Olaparib in novel oropharyngeal cancer cell lines infected with Human Papillomavirus
Abstract
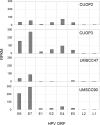
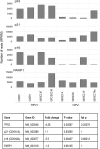
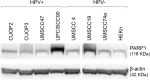
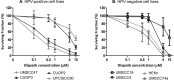
The incidence of Human Papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) is increasing rapidly in the UK. Patients with HPV-positive OPSCC generally show superior clinical responses relative to HPV-negative patients. We hypothesised that these superior responses could be associated with defective repair of DNA double strand breaks (DSB). The study aimed to determine whether defective DNA repair could be associated with sensitivity to inhibition of DNA repair using the PARP inhibitor Olaparib. Sensitivity to Olaparib, and induction and repair of DNA damage, were assessed in a panel of 8 OPSCC cell-lines, including 2 novel HPV-positive lines. Effects on cell cycle distribution and levels of PARP1 and p53 were quantified. RNA-sequencing was used to assess differences in activity of DNA repair pathways. Two HPV-positive OPSCC lines were sensitive to Olaparib at potentially therapeutic doses (0.1-0.5 μM). Two HPV-negative lines were sensitive at an intermediate dose. Four other lines, derived from HPV-positive and HPV-negative tumours, were resistant to PARP inhibition. Only one cell-line, UPCISCC90, showed results consistent with the original hypothesis i.e. that in HPV-positive cells, treatment with Olaparib would cause accumulation of DSB, resulting in cell cycle arrest. There was no evidence that HPV-positive tumours exhibit defective repair of DSB. However, the data suggest that a subset of OPSCC may be susceptible to PARP-inhibitor based therapy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Misregulation of DNA damage repair pathways in HPV-positive head and neck squamous cell carcinoma contributes to cellular radiosensitivity.Oncotarget. 2017 May 2;8(18):29963-29975. doi: 10.18632/oncotarget.16265. Oncotarget. 2017. PMID: 28415784 Free PMC article.
-
HPV-mediated PARP1 regulation and drug sensitization in head and neck cancer.Oral Oncol. 2025 Jun;165:107307. doi: 10.1016/j.oraloncology.2025.107307. Epub 2025 Apr 29. Oral Oncol. 2025. PMID: 40306238
-
Human papillomavirus DNA and p16 expression in Japanese patients with oropharyngeal squamous cell carcinoma.Cancer Med. 2013 Dec;2(6):933-41. doi: 10.1002/cam4.151. Epub 2013 Oct 27. Cancer Med. 2013. PMID: 24403267 Free PMC article.
-
Olaparib.Recent Results Cancer Res. 2018;211:217-233. doi: 10.1007/978-3-319-91442-8_15. Recent Results Cancer Res. 2018. PMID: 30069770 Review.
-
[From poly(ADP-ribose) discovery to PARP inhibitors in cancer therapy].Bull Cancer. 2015 Oct;102(10):863-73. doi: 10.1016/j.bulcan.2015.07.012. Epub 2015 Sep 15. Bull Cancer. 2015. PMID: 26384693 Review. French.
Cited by
-
RNA Sequencing and Cell Models of Virus-Associated Cancer (Review).Sovrem Tekhnologii Med. 2022;14(1):64-80. doi: 10.17691/stm2022.14.1.07. Epub 2022 Jan 28. Sovrem Tekhnologii Med. 2022. PMID: 35992999 Free PMC article. Review.
-
Targeted Therapy with PI3K, PARP, and WEE1 Inhibitors and Radiotherapy in HPV Positive and Negative Tonsillar Squamous Cell Carcinoma Cell Lines Reveals Synergy while Effects with APR-246 Are Limited.Cancers (Basel). 2022 Dec 23;15(1):93. doi: 10.3390/cancers15010093. Cancers (Basel). 2022. PMID: 36612094 Free PMC article.
-
Oral and Oropharyngeal Cancers and Possible Risk Factors Across Gulf Cooperation Council Countries: A Systematic Review.World J Oncol. 2020 Aug;11(4):173-181. doi: 10.14740/wjon1283. Epub 2020 Aug 10. World J Oncol. 2020. PMID: 32849958 Free PMC article.
-
A Lack of Effectiveness in the ATM-Orchestrated DNA Damage Response Contributes to the DNA Repair Defect of HPV-Positive Head and Neck Cancer Cells.Front Oncol. 2022 May 31;12:765968. doi: 10.3389/fonc.2022.765968. eCollection 2022. Front Oncol. 2022. PMID: 35719921 Free PMC article.
-
Pre-activation of autophagy impacts response to olaparib in prostate cancer cells.Commun Biol. 2022 Mar 22;5(1):251. doi: 10.1038/s42003-022-03210-5. Commun Biol. 2022. PMID: 35318456 Free PMC article.
References
-
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. (2013) Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 31: 4550–4559. 10.1200/JCO.2013.50.3870 - DOI - PMC - PubMed
-
- Marur S, D'Souza G, Westra WH, Forastiere AA (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11: 781–789. 10.1016/S1470-2045(10)70017-6 - DOI - PMC - PubMed
-
- Schache AG, Powell NG, Cuschieri KS, Robinson M, Leary S, Mehanna H, et al. (2016) HPV-Related Oropharynx Cancer in the United Kingdom: An Evolution in the Understanding of Disease Etiology. Cancer Res 76: 6598–6606. 10.1158/0008-5472.CAN-16-0633 - DOI - PMC - PubMed
-
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. (2010) Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med 363: 24–35. 10.1056/NEJMoa0912217 - DOI - PMC - PubMed
-
- Evans M, Newcombe R, Fiander A, Powell J, Rolles M, Thavaraj S, et al. (2013) Human Papillomavirus-associated oropharyngeal cancer: an observational study of diagnosis, prevalence and prognosis in a UK population. BMC Cancer 13: 220 10.1186/1471-2407-13-220 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

