Expression of the sRNAs CrcZ and CrcY modulate the strength of carbon catabolite repression under diazotrophic or non-diazotrophic growing conditions in Azotobacter vinelandii
- PMID: 30543677
- PMCID: PMC6292655
- DOI: 10.1371/journal.pone.0208975
Expression of the sRNAs CrcZ and CrcY modulate the strength of carbon catabolite repression under diazotrophic or non-diazotrophic growing conditions in Azotobacter vinelandii
Abstract
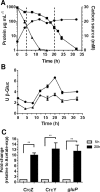
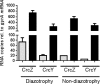
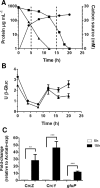
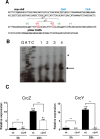
Azotobacter vinelandii is a nitrogen-fixing bacterium of the Pseudomonadaceae family that prefers the use of organic acids rather than carbohydrates. Thus, in a mixture of acetate-glucose, glucose is consumed only after acetate is exhausted. In a previous work, we investigated the molecular basis of this carbon catabolite repression (CCR) process under diazotrophic conditions. In the presence of acetate, Crc-Hfq inhibited translation of the gluP mRNA, encoding the glucose transporter in A. vinelandii. Herein, we investigated the regulation in the expression of the small non-coding RNAs (sRNAs) crcZ and crcY, which are known to antagonize the repressing activity of Hfq-Crc. Our results indicated higher expression levels of the sRNAs crcZ and crcY under low CCR conditions (i.e. glucose), in relation to the strong one (acetate one). In addition, we also explored the process of CCR in the presence of ammonium. Our results revealed that CCR also occurs under non-diazotrophic conditions as we detected a hierarchy in the utilization of the supplied carbon sources, which was consistent with the higher expression level of the crcZ/Y sRNAs during glucose catabolism. Analysis of the promoters driving transcription of crcZ and crcY confirmed that they were RpoN-dependent but we also detected a processed form of CrcZ (CrcZ*) in the RpoN-deficient strain derived from a cbrB-crcZ co-transcript. CrcZ* was functional and sufficient to allow the assimilation of acetate.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Two small RNAs, CrcY and CrcZ, act in concert to sequester the Crc global regulator in Pseudomonas putida, modulating catabolite repression.Mol Microbiol. 2012 Jan;83(1):24-40. doi: 10.1111/j.1365-2958.2011.07912.x. Epub 2011 Nov 20. Mol Microbiol. 2012. PMID: 22053874
-
The Crc/CrcZ-CrcY global regulatory system helps the integration of gluconeogenic and glycolytic metabolism in Pseudomonas putida.Environ Microbiol. 2015 Sep;17(9):3362-78. doi: 10.1111/1462-2920.12812. Epub 2015 Mar 27. Environ Microbiol. 2015. PMID: 25711694
-
Effect of Crc and Hfq proteins on the transcription, processing, and stability of the Pseudomonas putida CrcZ sRNA.RNA. 2016 Dec;22(12):1902-1917. doi: 10.1261/rna.058313.116. Epub 2016 Oct 24. RNA. 2016. PMID: 27777366 Free PMC article.
-
Rewiring the functional complexity between Crc, Hfq and sRNAs to regulate carbon catabolite repression in Pseudomonas.World J Microbiol Biotechnol. 2019 Aug 26;35(9):140. doi: 10.1007/s11274-019-2717-7. World J Microbiol Biotechnol. 2019. PMID: 31451938 Review.
-
Carbohydrate Utilization in Bacteria: Making the Most Out of Sugars with the Help of Small Regulatory RNAs.Microbiol Spectr. 2018 Mar;6(2):10.1128/microbiolspec.rwr-0013-2017. doi: 10.1128/microbiolspec.RWR-0013-2017. Microbiol Spectr. 2018. PMID: 29573258 Free PMC article. Review.
Cited by
-
Fructose promotes pyoluteorin biosynthesis via the CbrAB-CrcZ-Hfq/Crc pathway in the biocontrol strain Pseudomonas PA1201.Synth Syst Biotechnol. 2023 Sep 21;8(4):618-628. doi: 10.1016/j.synbio.2023.09.004. eCollection 2023 Dec. Synth Syst Biotechnol. 2023. PMID: 37823038 Free PMC article.
-
Regulation of hierarchical carbon substrate utilization, nitrogen fixation, and root colonization by the Hfq/Crc/CrcZY genes in Pseudomonas stutzeri.iScience. 2022 Nov 23;25(12):105663. doi: 10.1016/j.isci.2022.105663. eCollection 2022 Dec 22. iScience. 2022. PMID: 36505936 Free PMC article.
-
The Modification of Regulatory Circuits Involved in the Control of Polyhydroxyalkanoates Metabolism to Improve Their Production.Front Bioeng Biotechnol. 2020 Apr 30;8:386. doi: 10.3389/fbioe.2020.00386. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32426348 Free PMC article. Review.
-
The Regulatory Hierarchy Following Signal Integration by the CbrAB Two-Component System: Diversity of Responses and Functions.Genes (Basel). 2022 Feb 18;13(2):375. doi: 10.3390/genes13020375. Genes (Basel). 2022. PMID: 35205417 Free PMC article. Review.
-
Implications of carbon catabolite repression for plant-microbe interactions.Plant Commun. 2021 Dec 28;3(2):100272. doi: 10.1016/j.xplc.2021.100272. eCollection 2022 Mar 14. Plant Commun. 2021. PMID: 35529946 Free PMC article. Review.
References
-
- Segura D, Núñez C, Espín G. Azotobacter cysts eLS Wiley Online Library; 2014.
-
- Kennedy C, Rudnick P, MacDonald ML, Melton T. Genus III. Azotobacter Beijerinck In: 1901 AIBD, Noel RK, Staley JT and Garrity GM (eds) editor. Bergey’s Manual of Systematic Bacteriology The Proteobacteria, pp.: New York, NY: Springer.; 2005. p. 384–402.
-
- Quiroz-Rocha E, Moreno R, Hernández-Ortiz A, Fragoso-Jiménez JC, Muriel-Millán LF, Guzmán J, et al. Glucose uptake in Azotobacter vinelandii occurs through a GluP transporter that is under the control of the CbrA/CbrB and Hfq-Crc systems. Scientific reports. 2017;7(1):858 10.1038/s41598-017-00980-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

