Sphingosine 1-phosphate (S1P) reduces hepatocyte growth factor-induced migration of hepatocellular carcinoma cells via S1P receptor 2
- PMID: 30543684
- PMCID: PMC6292590
- DOI: 10.1371/journal.pone.0209050
Sphingosine 1-phosphate (S1P) reduces hepatocyte growth factor-induced migration of hepatocellular carcinoma cells via S1P receptor 2
Abstract
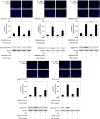
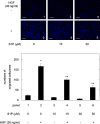
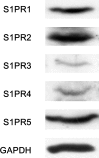
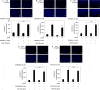
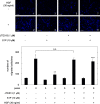
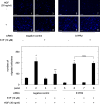
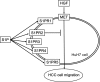
A bioactive lipid, sphingosine 1-phosphate (S1P), acts extracellularly as a potent mediator, and is implicated in the progression of various cancers including hepatocellular carcinoma (HCC). S1P exerts its functions by binding to five types of specific receptors, S1P receptor 1 (S1PR1), S1PR2, S1PR3, S1PR4 and S1PR5 on the plasma membrane. However, the exact roles of S1P and each S1PR in HCC cells remain to be clarified. In the present study, we investigated the effect of S1P on the hepatocyte growth factor (HGF)-induced migration of human HCC-derived HuH7 cells, and the involvement of each S1PR. S1P dose-dependently reduced the HGF-induced migration of HuH7 cells. We found that all S1PRs exist in the HuH7 cells. Among each selective agonist for five S1PRs, CYM5520, a selective S1PR2 agonist, significantly suppressed the HGF-induced HuH7 cell migration whereas selective agonists for S1PR1, S1PR3, S1PR4 or S1PR5 failed to affect the migration. The reduction of the HGF-induced migration by S1P was markedly reversed by treatment of JTE013, a selective antagonist for S1PR2, and S1PR2- siRNA. These results strongly suggest that S1P reduces the HGF-induced HCC cell migration via S1PR2. Our findings may provide a novel potential of S1PR2 to therapeutic strategy for metastasis of HCC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Interaction of integrin β4 with S1P receptors in S1P- and HGF-induced endothelial barrier enhancement.J Cell Biochem. 2014 Jun;115(6):1187-95. doi: 10.1002/jcb.24770. J Cell Biochem. 2014. PMID: 24851274 Free PMC article.
-
Follicular fluid high-density lipoprotein-associated sphingosine 1-phosphate (S1P) promotes human granulosa lutein cell migration via S1P receptor type 3 and small G-protein RAC1.Biol Reprod. 2011 Mar;84(3):604-12. doi: 10.1095/biolreprod.110.084152. Epub 2010 Oct 27. Biol Reprod. 2011. PMID: 20980685
-
Essential roles of sphingosine 1-phosphate receptor types 1 and 3 in human hepatic stellate cells motility and activation.J Cell Physiol. 2011 Sep;226(9):2370-7. doi: 10.1002/jcp.22572. J Cell Physiol. 2011. PMID: 21660960
-
Sphingosine-1-phosphate receptor 2.FEBS J. 2013 Dec;280(24):6354-66. doi: 10.1111/febs.12446. Epub 2013 Aug 19. FEBS J. 2013. PMID: 23879641 Free PMC article. Review.
-
Sphingosine-1-phosphate/sphingosine-1-phosphate receptor 1 and T cell migration.Yao Xue Xue Bao. 2016 Jun;51(6):873-8. Yao Xue Xue Bao. 2016. PMID: 29878740 Review.
Cited by
-
PDZK1 inhibits MRP2-mediated oxaliplatin chemosensitivity in hepatocellular carcinoma.Sci Rep. 2025 Apr 18;15(1):13438. doi: 10.1038/s41598-025-98085-x. Sci Rep. 2025. PMID: 40251307 Free PMC article.
-
Targeting SPHK1/S1PR3-regulated S-1-P metabolic disorder triggers autophagic cell death in pulmonary lymphangiomyomatosis (LAM).Cell Death Dis. 2022 Dec 21;13(12):1065. doi: 10.1038/s41419-022-05511-3. Cell Death Dis. 2022. PMID: 36543771 Free PMC article.
-
Role of S1PR1 in VEGF-exosomes mediated resistance of hepatocellular carcinoma to anti-angiogenesis therapy.Cancer Cell Int. 2025 Jul 16;25(1):264. doi: 10.1186/s12935-025-03907-7. Cancer Cell Int. 2025. PMID: 40671013 Free PMC article.
-
Heat shock protein 70 positively regulates transforming growth factor-α-induced hepatocellular carcinoma cell migration via the AKT signaling pathway.Heliyon. 2020 Sep 23;6(9):e05002. doi: 10.1016/j.heliyon.2020.e05002. eCollection 2020 Sep. Heliyon. 2020. PMID: 33005803 Free PMC article.
-
Interaction of microRNAs with sphingosine kinases, sphingosine-1 phosphate, and sphingosine-1 phosphate receptors in cancer.Discov Oncol. 2021 Sep 20;12(1):33. doi: 10.1007/s12672-021-00430-9. Discov Oncol. 2021. PMID: 35201458 Free PMC article. Review.
References
-
- Liovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016; 2: 1–23. - PubMed
-
- Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastasis. J Gastroenterol Hepatol. 2005; 20: 1781–1787. 10.1111/j.1440-1746.2005.03919.x - DOI - PubMed
-
- Sneag DB, Krajewski K, Giardino A, O’Regan KN, Shinagare AB, Jagannathan JP, et al. Extrahepatic spread or hepatocellular carcinoma: Spectrum of imaging findings. AJR Am J Roentgenol. 2011; 197: W658–W664. 10.2214/AJR.10.6402 - DOI - PubMed
-
- Poon RTP, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation. Impact on long-term survival. Ann Surg. 2007; 245: 51–58. 10.1097/01.sla.0000225255.01668.65 - DOI - PMC - PubMed
-
- Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: Five steps to prevent recurrence. Am J Transplant. 2011; 11: 2031–2035. 10.1111/j.1600-6143.2011.03689.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

