Endogenous insulin signaling in the RPE contributes to the maintenance of rod photoreceptor function in diabetes
- PMID: 30543793
- PMCID: PMC6389378
- DOI: 10.1016/j.exer.2018.11.020
Endogenous insulin signaling in the RPE contributes to the maintenance of rod photoreceptor function in diabetes
Abstract
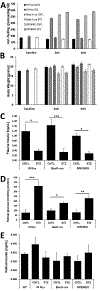
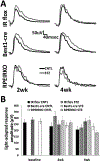
In diabetes, there are two major physiological aberrations: (i) Loss of insulin signaling due to absence of insulin (type 1 diabetes) or insulin resistance (type 2 diabetes) and (ii) increased blood glucose levels. The retina has a high proclivity to damage following diabetes, and much of the pathology seen in diabetic retinopathy has been ascribed to hyperglycemia and downstream cascades activated by increased blood glucose. However, less attention has been focused on the direct role of insulin on retinal physiology, likely due to the fact that uptake of glucose in retinal cells is not insulin-dependent. The retinal pigment epithelium (RPE) is instrumental in maintaining the structural and functional integrity of the retina. Recent studies have suggested that RPE dysfunction is a precursor of, and contributes to, the development of diabetic retinopathy. To evaluate the role of insulin on RPE cell function directly, we generated a RPE specific insulin receptor (IR) knockout (RPEIRKO) mouse using the Cre-loxP system. Using this mouse, we sought to determine the impact of insulin-mediated signaling in the RPE on retinal function under physiological control conditions as well as in streptozotocin (STZ)-induced diabetes. We demonstrate that loss of RPE-specific IR expression resulted in lower a- and b-wave electroretinogram amplitudes in diabetic mice as compared to diabetic mice that expressed IR on the RPE. Interestingly, RPEIRKO mice did not exhibit significant differences in the amplitude of the RPE-dependent electroretinogram c-wave as compared to diabetic controls. However, loss of IR-mediated signaling in the RPE reduced levels of reactive oxygen species and the expression of pro-inflammatory cytokines in the retina of diabetic mice. These results imply that IR-mediated signaling in the RPE regulates photoreceptor function and may play a role in the generation of oxidative stress and inflammation in the retina in diabetes.
Keywords: Diabetic retinopathy; Insulin; Oxidative stress; Photoreceptor; Retinal pigment epithelium.
Published by Elsevier Ltd.
Figures
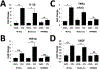




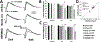



Similar articles
-
Early retinal pigment epithelium dysfunction is concomitant with hyperglycemia in mouse models of type 1 and type 2 diabetes.J Neurophysiol. 2015 Feb 15;113(4):1085-99. doi: 10.1152/jn.00761.2014. Epub 2014 Nov 26. J Neurophysiol. 2015. PMID: 25429122 Free PMC article.
-
Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line.Diabetologia. 2011 Jun;54(6):1567-78. doi: 10.1007/s00125-011-2061-4. Epub 2011 Mar 4. Diabetologia. 2011. PMID: 21373835 Free PMC article.
-
Reduction of Glut1 in the Neural Retina But Not the RPE Alleviates Polyol Accumulation and Normalizes Early Characteristics of Diabetic Retinopathy.J Neurosci. 2021 Apr 7;41(14):3275-3299. doi: 10.1523/JNEUROSCI.2010-20.2021. Epub 2021 Feb 23. J Neurosci. 2021. PMID: 33622781 Free PMC article.
-
The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy.J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. Epub 2010 Feb 17. J Biomed Biotechnol. 2010. PMID: 20182540 Free PMC article. Review.
-
Photoreceptor cells and RPE contribute to the development of diabetic retinopathy.Prog Retin Eye Res. 2021 Jul;83:100919. doi: 10.1016/j.preteyeres.2020.100919. Epub 2020 Nov 12. Prog Retin Eye Res. 2021. PMID: 33188897 Free PMC article. Review.
Cited by
-
Towards a New Biomarker for Diabetic Retinopathy: Exploring RBP3 Structure and Retinoids Binding for Functional Imaging of Eyes In Vivo.Int J Mol Sci. 2023 Feb 23;24(5):4408. doi: 10.3390/ijms24054408. Int J Mol Sci. 2023. PMID: 36901838 Free PMC article. Review.
-
Transcriptome Analyses of lncRNAs in A2E-Stressed Retinal Epithelial Cells Unveil Advanced Links between Metabolic Impairments Related to Oxidative Stress and Retinitis Pigmentosa.Antioxidants (Basel). 2020 Apr 15;9(4):318. doi: 10.3390/antiox9040318. Antioxidants (Basel). 2020. PMID: 32326576 Free PMC article.
-
Mitophagy, Ferritinophagy and Ferroptosis in Retinal Pigment Epithelial Cells Under High Glucose Conditions: Implications for Diabetic Retinopathy and Age-Related Retinal Diseases.JOJ Ophthalmol. 2021;8(5):77-85. Epub 2021 Sep 27. JOJ Ophthalmol. 2021. PMID: 35187384 Free PMC article.
-
Diet, Mitochondrial Dysfunction, Vascular Endothelial Damage, and the Microbiome: Drivers of Ocular Degenerative and Inflammatory Diseases.Ophthalmol Ther. 2025 Jul;14(7):1429-1452. doi: 10.1007/s40123-025-01160-9. Epub 2025 May 28. Ophthalmol Ther. 2025. PMID: 40434533 Free PMC article. Review.
-
Phagocytosis in the retina promotes local insulin production in the eye.Nat Metab. 2023 Feb;5(2):207-218. doi: 10.1038/s42255-022-00728-0. Epub 2023 Feb 2. Nat Metab. 2023. PMID: 36732622 Free PMC article.
References
-
- Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW, 2001. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. The Journal of biological chemistry 276, 32814–32821. - PubMed
-
- Chan EC, van Wijngaarden P, Liu GS, Jiang F, Peshavariya H, Dusting GJ, 2013. Involvement of Nox2 NADPH oxidase in retinal neovascularization. Investigative ophthalmology & visual science 54, 7061–7067. - PubMed
-
- Chong MP, Barritt GJ, Crouch MF, 2004. Insulin potentiates EGFR activation and signaling in fibroblasts. Biochemical and biophysical research communications 322, 535–541. - PubMed
-
- Haussinger D, Lang F, 1992. Cell volume and hormone action. Trends in pharmacological sciences 13, 371–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

