Trends in Opioid Prescribing Among Hemodialysis Patients, 2007-2014
- PMID: 30544114
- PMCID: PMC6341485
- DOI: 10.1159/000495353
Trends in Opioid Prescribing Among Hemodialysis Patients, 2007-2014
Abstract
Background: Hemodialysis (HD) patients frequently experience pain. Previous studies of HD patients suggest increased opioid prescribing through 2010. It remains unclear if this trend continued after 2010 or declined with national trends.
Methods: Longitudinal cohort study of 484,745 HD patients in the United States Renal Data System/Medicare data. We used Poisson/negative binomial regression to estimate annual incidence rates of opioid prescribing between 2007 and 2014. We compared prescribing rates with the general US population using IQVIA's National Prescription Audit data. Outcomes included the following: percent of HD patients receiving an opioid prescription, rate of opioid prescriptions, quantity, days supply, morphine milligram equivalents (MME) dispensed per 100 person-days, and prescriptions per person.
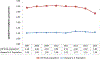
Results: In 2007, 62.4% of HD patients received an opioid prescription. This increased to 63.2% in 2010 then declined to 53.7% by 2014. Opioid quantity peaked in 2011 at 73.5 pills per 100 person-days and declined to 62.6 pills per 100 person-days in 2014. MME peaked between 2010 and 2012 then declined through 2014. In 2014, MME rates were 1.8-fold higher among non-Hispanic patients and 1.6-fold higher among low-income patients. HD patients received 3.2-fold more opioid prescriptions per person compared to the general US population and were primarily prescribed oxycodone and hydrocodone. Between 2012 and 2014, HD patients experienced greater declines in opioid prescriptions per person (18.2%) compared to the general US population (7.1%).
Conclusion: Opioid prescribing among HD patients declined between 2012 and 2014. However, HD patients continue receiving substantially more opioids than the general US population.
Keywords: Hemodialysis; Opioid prescribing; Trends; United States.
© 2018 S. Karger AG, Basel.
Conflict of interest statement
Disclosure statement
Dr. Alexander is Chair of the FDA’s Peripheral and Central Nervous System Advisory Committee; serves on the Advisory Board of MesaRx Innovations; holds equity in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and serves as a member of OptumRx’s P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. Remaining authors have no conflicts of interest to report.
Figures

References
-
- U.S. Agency for Health Care Policy and Research. Acute Pain Management: Operative or Medical Procedures and Trauma. (AHCPR Pub. No. 92–0032) Rockville, MD, U.S. Department of Health and Human Services, 1992.
-
- American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 4 Glenview, IL: American Pain Society; 1999.
-
- American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 1995;274:1874–80. - PubMed
-
- World Health Organization. WHO’s Pain Relief Ladder (2009). Available at: www.who.int/cancer/palliative/painladder/en/ (Accessed January 2, 2017).
-
- Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers---United States, 1999−−2008. MMWR Morb Mortal Wkly Rep 2011. November 4;60(43):1487–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

