Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo
- PMID: 30544191
- PMCID: PMC6379722
- DOI: 10.1093/nar/gky1239
Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo
Abstract
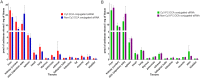
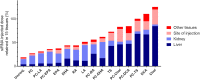
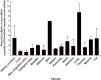
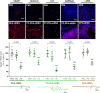
Small interfering RNA (siRNA)-based therapies are proving to be efficient for treating liver-associated disorders. However, extra-hepatic delivery remains challenging, limiting therapeutic siRNA utility. We synthesized a panel of fifteen lipid-conjugated siRNAs and systematically evaluated the impact of conjugate on siRNA tissue distribution and efficacy. Generally, conjugate hydrophobicity defines the degree of clearance and the liver-to-kidney distribution profile. In addition to primary clearance tissues, several conjugates achieve significant siRNA accumulation in muscle, lung, heart, adrenal glands and fat. Oligonucleotide distribution to extra-hepatic tissues with some conjugates was significantly higher than with cholesterol, a well studied conjugate, suggesting that altering conjugate structure can enhance extra-hepatic delivery. These conjugated siRNAs enable functional gene silencing in lung, muscle, fat, heart and adrenal gland. Required levels for productive silencing vary (5-200 μg/g) per tissue, suggesting that the chemical nature of conjugates impacts tissue-dependent cellular/intracellular trafficking mechanisms. The collection of conjugated siRNA described here enables functional gene modulation in vivo in several extra-hepatic tissues opening these tissues for gene expression modulation. A systemic evaluation of a panel of conjugated siRNA, as reported here, has not previously been investigated and shows that chemical engineering of lipid siRNAs is essential to advance the RNA therapeutic field.
© The Author(s) 2018. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
-
- Nair J.K., Willoughby J.L.S., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S. et al. . Multivalent N-Acetylgalactosamine-Conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. JACS. 2014; 136:16958–16961. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

