Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma
- PMID: 30544335
- PMCID: PMC7266009
- DOI: 10.3171/2018.9.PEDS17225
Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma
Abstract
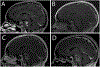
OBJECTIVE In this clinical trial report, the authors analyze safety and infusion distribution of IL13-Pseudomonas exotoxin, an antitumor chimeric molecule, administered via intratumoral convection enhanced delivery (CED) in pediatric patients with diffuse intrinsic pontine glioma (DIPG). METHODS This was a Phase I single-institution, open-label, dose-escalation, safety and tolerability study of IL13-PE38QQR infused via single-catheter CED into 5 pediatric DIPG patients. IL13-PE38QQR was administered to regions of tumor selected by radiographic findings. Two escalating dose levels were evaluated: 0.125 µg/mL in cohort 1 and 0.25 µg/mL in cohort 2. Real-time MRI was performed during intratumoral infusions, and MRI and MR spectroscopy were performed before and after the infusions. Clinical evaluations, including parent-reported quality of life (QOL), were assessed at baseline and 4 weeks post-infusion. RESULTS Direct infusion of brainstem tumor with IL13-PE using the CED technique in patients with DIPG produced temporary arrest of disease progression in 2 of 5 patients, both of whom subsequently received a second infusion. All 5 patients showed signs of disease progression by 12 weeks after initial infusion. Two patients experienced transient cranial nerve deficits and lethargy after infusion, and these deficits resolved with corticosteroid treatment in both cases. No patient had radiographic evidence of acute or long-term treatment toxicity. Parent-reported QOL was consistent with medical outcomes. CONCLUSIONS Even though IL13-PE delivered by CED did not reach the entire MRI-defined tumor volume in any patient, short-term radiographic antitumor effects were observed in 2 of the 5 patients treated. The patients’ performance status did not improve. Drug delivery using multiple catheters may produce improved outcomes. Clinical trial registration no.: NCT00088061 (clinicaltrials.gov) ABBREVIATIONS CED = convection-enhanced delivery; DIPG = diffuse intrinsic pontine glioma; IL-13 = interleukin 13; IL13R = IL-13 receptor; IPI = Impact of Pediatric Illness; KPS = Karnofsky Performance Status; LPS = Lansky Performance Status; MRS = MR spectroscopy; NAA = n-acetyl aspartate; QOL = quality of life; Vd = volume of distribution; Vi = volume of infusion.
Keywords: CED; CED = convection-enhanced delivery; DIPG; DIPG = diffuse intrinsic pontine glioma; Gd-DTPA; IL-13 = interleukin 13; IL13-PE; IL13R = IL-13 receptor; IPI = Impact of Pediatric Illness; KPS = Karnofsky Performance Status; LPS = Lansky Performance Status; MR-spectroscopy; MRS = MR spectroscopy; NAA = n-acetyl aspartate; QOL = quality of life; Vd = volume of distribution; Vi = volume of infusion; convection-enhanced delivery; oncology; pediatric brain tumors.
Conflict of interest statement
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Figures




References
-
- Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD: Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery 33:1026–1030, 1993 - PubMed
-
- Broniscer A, Gajjar A: Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist 9:197–206, 2004 - PubMed
-
- Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY: Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res 5:985–990, 1999 - PubMed
-
- Debinski W, Obiri NI, Pastan I, Puri RK: A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem 270:16775–16780, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

