MET/HGF Co-Targeting in Pancreatic Cancer: A Tool to Provide Insight into the Tumor/Stroma Crosstalk
- PMID: 30544501
- PMCID: PMC6321305
- DOI: 10.3390/ijms19123920
MET/HGF Co-Targeting in Pancreatic Cancer: A Tool to Provide Insight into the Tumor/Stroma Crosstalk
Abstract
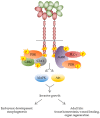
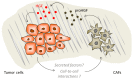
The 'onco-receptor' MET (Hepatocyte Growth Factor Receptor) is involved in the activation of the invasive growth program that is essential during embryonic development and critical for wound healing and organ regeneration during adult life. When aberrantly activated, MET and its stroma-secreted ligand HGF (Hepatocyte Growth Factor) concur to tumor onset, progression, and metastasis in solid tumors, thus representing a relevant target for cancer precision medicine. In the vast majority of tumors, wild-type MET behaves as a 'stress-response' gene, and relies on ligand stimulation to sustain cancer cell 'scattering', invasion, and protection form apoptosis. Moreover, the MET/HGF axis is involved in the crosstalk between cancer cells and the surrounding microenvironment. Pancreatic cancer (namely, pancreatic ductal adenocarcinoma, PDAC) is an aggressive malignancy characterized by an abundant stromal compartment that is associated with early metastases and resistance to conventional and targeted therapies. Here, we discuss the role of the MET/HGF axis in tumor progression and dissemination considering as a model pancreatic cancer, and provide a proof of concept for the application of dual MET/HGF inhibition as an adjuvant therapy in pancreatic cancer patients.
Keywords: HGF; MET; metastasis; pancreatic cancer; target therapy; tumor microenvironment.
Conflict of interest statement
P.M.C. and E.V. are co-founders of Metis Precision Medicine B-Corp (Italy). The company did not interfere in the writing of the manuscript and in the decision to publish. The other authors declare no conflict of interest.
Figures


References
-
- Naldini L., Weidner K.M., Vigna E., Gaudino G., Bardelli A., Ponzetto C., Narsimhan R.P., Hartmann G., Zarnegar R., Michalopoulos G.K. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

