Fluorescent Aptamer Immobilization on Inverse Colloidal Crystals
- PMID: 30544583
- PMCID: PMC6308693
- DOI: 10.3390/s18124326
Fluorescent Aptamer Immobilization on Inverse Colloidal Crystals
Abstract
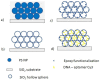
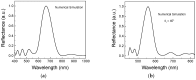
In this paper, we described a versatile two steps approach for the realization of silica inverse opals functionalized with DNA-aptamers labelled with Cy3 fluorophore. The co-assembly method was successfully employed for the realization of high quality inverse silica opal, whilst the inverse network was functionalized via epoxy chemistry. Morphological and optical assessment revealed the presence of large ordered domains with a transmission band gap depth of 32%, after the functionalization procedure. Finite Difference Time-Domain (FDTD) simulations confirmed the high optical quality of the inverse opal realized. Photoluminescence measurements evidenced the effective immobilization of DNA-aptamer molecules labelled with Cy3 throughout the entire sample thickness. This assumption was verified by the inhibition of the fluorescence of Cy3 fluorophore tailoring the position of the photonic band gap of the inverse opal. The modification of the fluorescence could be justified by a variation in the density of states (DOS) calculated by the Plane Wave Expansion (PWE) method. Finally, the development of the aforementioned approach could be seen as proof of the concept experiment, suggesting that this type of system may act as a suitable platform for the realization of fluorescence-based bio-sensors.
Keywords: DNA-aptamers; FDTD simulations; PWE method; band gap; co-assembly; colloidal crystal; fluorescence.
Conflict of interest statement
The authors declare no conflicts of interest
Figures





References
-
- Singh P. SPR Biosensors: Historical Perspectives and Current Challenges. Sens. Actuator B-Chem. 2016;229:110–130. doi: 10.1016/j.snb.2016.01.118. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

