Modulation of Protein Synthesis by eIF2α Phosphorylation Protects Cell from Heat Stress-Mediated Apoptosis
- PMID: 30544621
- PMCID: PMC6316477
- DOI: 10.3390/cells7120254
Modulation of Protein Synthesis by eIF2α Phosphorylation Protects Cell from Heat Stress-Mediated Apoptosis
Abstract
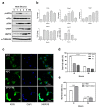
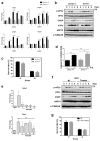
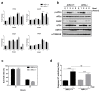
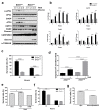
Global warming poses a considerable threat to human health, necessitating a proper understanding of mechanisms underlying cell death in the pathogenesis of heat-related diseases. Although mechanisms governing cytoplasmic response to heat are well understood, processes regulating cellular response to disruption of proteostasis in the endoplasmic reticulum (ER) due to heat stress remain unclear. The current study reveals that hyperthermic conditions may lead to a disturbance of ER homeostasis, also known as ER stress. Subsequent activation of the unfolded protein response (UPR) resulted in concomitant induction of cell death. Among the three UPR signaling pathways, the eIF2α phosphorylation pathway, and not the IRE1α/ATF6α pathways, is likely the main contributor to cell death under heat stress. Considering the role of eIF2α in translational control, we investigated the protective effect of translation rate on heat stress-mediated cell death. When protein synthesis was attenuated using cycloheximide or homoharringtonine, cell death due to heat stress was significantly reduced. In summation, we propose that transient modulation of protein synthesis by eIF2α phosphorylation has a pivotal role in protecting cells from heat stress-induced apoptosis. Therefore, pharmacological agents that promote eIF2α phosphorylation or reduce ER stress may contribute to the development of promising therapeutic approaches against heat-related diseases.
Keywords: ER stress; eIF2α phosphorylation; heat stress; translation; unfolded protein response (UPR).
Conflict of interest statement
All the authors declare no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

