Generation of A Mucor circinelloides Reporter Strain-A Promising New Tool to Study Antifungal Drug Efficacy and Mucormycosis
- PMID: 30544643
- PMCID: PMC6315630
- DOI: 10.3390/genes9120613
Generation of A Mucor circinelloides Reporter Strain-A Promising New Tool to Study Antifungal Drug Efficacy and Mucormycosis
Abstract
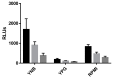
Invasive fungal infections caused by Mucorales (mucormycosis) have increased worldwide. These life-threatening infections affect mainly, but not exclusively, immunocompromised patients, and are characterized by rapid progression, severe tissue damage and an unacceptably high rate of mortality. Still, little is known about this disease and its successful therapy. New tools to understand mucormycosis and a screening method for novel antimycotics are required. Bioluminescent imaging is a powerful tool for in vitro and in vivo approaches. Hence, the objective of this work was to generate and functionally analyze bioluminescent reporter strains of Mucor circinelloides, one mucormycosis-causing pathogen. Reporter strains were constructed by targeted integration of the firefly luciferase gene under control of the M. circinelloides promoter Pzrt1. The luciferase gene was sufficiently expressed, and light emission was detected under several conditions. Phenotypic characteristics, virulence potential and antifungal susceptibility were indifferent to the wild-type strains. Light intensity was dependent on growth conditions and biomass, being suitable to determine antifungal efficacy in vitro. This work describes for the first time the generation of reporter strains in a basal fungus that will allow real-time, non-invasive infection monitoring in insect and murine models, and the testing of antifungal efficacy by means other than survival.
Keywords: Mucor circinelloides; bioluminescence; firefly luciferase; mucormycosis; reporter strain.
Conflict of interest statement
C.L.-F. has received grant support from the Austrian Science Fund (FWF), MFF Tirol, Astellas Pharma, Gilead Sciences, Pfizer, Schering Plough, and Merck Sharp & Dohme. She has been an advisor/consultant to Gilead Sciences, Merck Sharp & Dohme, Pfizer, and Schering Plough. She has received travel/accommodation expenses from Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas, and Schering Plough and has been paid for talks on behalf of Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas, and Schering Plough. All other authors declare no conflict of interest.
Figures




References
-
- Ingold C.T. The Biology of Mucor and Its Allies. E. Arnold; London, UK: 1978. Studies in biology no. 88.
-
- Kontoyiannis D.P., Lionakis M.S., Lewis R.E., Chamilos G., Healy M., Perego C., Safdar A., Kantarjian H., Champlin R., Walsh T.J., et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: A case-control observational study of 27 recent cases. J. Infect. Dis. 2005;191:1350–1360. doi: 10.1086/428780. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

