DNA Damage Tolerance Mechanisms Revealed from the Analysis of Immunoglobulin V Gene Diversification in Avian DT40 Cells
- PMID: 30544644
- PMCID: PMC6316486
- DOI: 10.3390/genes9120614
DNA Damage Tolerance Mechanisms Revealed from the Analysis of Immunoglobulin V Gene Diversification in Avian DT40 Cells
Abstract
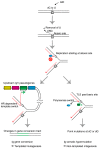
DNA replication is an essential biochemical reaction in dividing cells that frequently stalls at damaged sites. Homologous/homeologous recombination (HR)-mediated template switch and translesion DNA synthesis (TLS)-mediated bypass processes release arrested DNA replication forks. These mechanisms are pivotal for replication fork maintenance and play critical roles in DNA damage tolerance (DDT) and gap-filling. The avian DT40 B lymphocyte cell line provides an opportunity to examine HR-mediated template switch and TLS triggered by abasic sites by sequencing the constitutively diversifying immunoglobulin light-chain variable gene (IgV). During IgV diversification, activation-induced deaminase (AID) converts dC to dU, which in turn is excised by uracil DNA glycosylase and yields abasic sites within a defined window of around 500 base pairs. These abasic sites can induce gene conversion with a set of homeologous upstream pseudogenes via the HR-mediated template switch, resulting in templated mutagenesis, or can be bypassed directly by TLS, resulting in non-templated somatic hypermutation at dC/dG base pairs. In this review, we discuss recent works unveiling IgV diversification mechanisms in avian DT40 cells, which shed light on DDT mode usage in vertebrate cells and tolerance of abasic sites.
Keywords: DNA damage; abasic site; activation-induced deaminase; homologous recombination; replication; translesion DNA synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Friedberg E.C., Walker G.C., Siede W., Wood R.D., Schultz R.A., Ellenberger T. DNA Repair and Mutagenesis. ASM Press; Boston, MA, USA: 2006.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

