Rhabdomyosarcoma and Extraosseous Ewing Sarcoma
- PMID: 30544742
- PMCID: PMC6306718
- DOI: 10.3390/children5120165
Rhabdomyosarcoma and Extraosseous Ewing Sarcoma
Abstract
Rhabdomyosarcoma (RMS) is a malignant tumor that represents the most common form of pediatric soft tissue sarcoma. It arises from mesenchymal origin and forms part of the group of small round blue cell tumors of childhood. It has a constant annual incidence of 4.5 cases per 1,000,000 children. The known histological diagnosis of the two major subtypes (embryonal and alveolar) has been recently enhanced by tumor biological markers and molecular differentiation diagnostic tools that have improved not only the updated classification based on risk stratification, but also the treatment approach based on the clinical group. Ewing sarcoma (ES) is a round cell tumor, highly malignant and poorly differentiated that is currently the second most common malignant bone tumor in children. In rare instances, it develops from an extraskeletal origin, classified as extraosseous Ewing sarcoma (EES). We provide an updated, evidence-based and comprehensive review of the molecular diagnosis, clinical and diagnostic approach and a multidisciplinary medical and surgical management according to the latest standard of care for the treatment of pediatric RMS and EES.
Keywords: extraosseous Ewing sarcoma; pediatric; rhabdomyosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
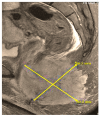
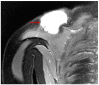
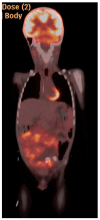
Figures





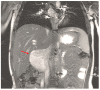
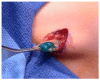
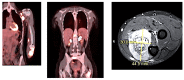
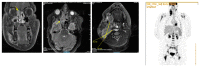
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

