Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies
- PMID: 30544808
- PMCID: PMC6316801
- DOI: 10.3390/nu10121950
Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies
Abstract
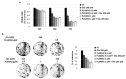
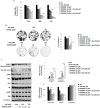
Oleuropein (Ole), a secoiridoid glucoside present in Olea europaea leaves, gained scientific interest thanks to its several biological properties, including the anticancer one. We verified whether Ole might potentiate the cytotoxicity of conventional drugs used to treat melanoma, disclosing a potentially new therapeutic strategy. We tested the cytotoxic action of Ole alone or in combination with chemotherapeutics on A375 human melanoma cells. We found that Ole was able, at a dose of 500 µM, to stimulate apoptosis, while at a non-toxic dose of 250 µM, it affected cell proliferation and induced the downregulation of the pAKT/pS6 pathway. A dose of 250 µM Ole did not potentiate the effect of Vemurafenib (PLX4032), but it succeeded in increasing the cytotoxic effect of Dacarbazine (DTIC). The major effect was found in the association between Ole and Everolimus (RAD001), also on PLX4032-resistant BRAF melanoma cells, which possibly cooperate in the inhibition of the pAKT/pS6 pathway. Of interest, an olive leaf extract enriched in equimolar Ole was more effective and able to further improve DTIC and RAD001 efficacy on BRAF melanoma cells with respect to Ole alone. Therefore, Ole represents a natural product able to potentiate a wide array of chemotherapeutics against BRAF melanoma cells affecting the pAKT/pS6 pathway.
Keywords: BRAF melanoma; Oleuropein; chemotherapeutics; extra virgin oil; olive leaf extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wan P.T.C., Garnett M.J., Roe S.M., Lee S., Niculescu-Duvaz D., Good V.M., Jones C.M., Marshall C.J., Springer C.J., Barford D., et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/S0092-8674(04)00215-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

