Chromatin as a Platform for Modulating the Replication Stress Response
- PMID: 30544989
- PMCID: PMC6316668
- DOI: 10.3390/genes9120622
Chromatin as a Platform for Modulating the Replication Stress Response
Abstract
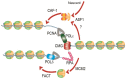
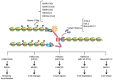
Eukaryotic DNA replication occurs in the context of chromatin. Recent years have seen major advances in our understanding of histone supply, histone recycling and nascent histone incorporation during replication. Furthermore, much is now known about the roles of histone remodellers and post-translational modifications in replication. It has also become clear that nucleosome dynamics during replication play critical roles in genome maintenance and that chromatin modifiers are important for preventing DNA replication stress. An understanding of how cells deploy specific nucleosome modifiers, chaperones and remodellers directly at sites of replication fork stalling has been building more slowly. Here we will specifically discuss recent advances in understanding how chromatin composition contribute to replication fork stability and restart.
Keywords: chromatin; chromatin remodeller; genome instability; histone chaperone; replication stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

