Oleiferasaponin A₂, a Novel Saponin from Camellia oleifera Abel. Seeds, Inhibits Lipid Accumulation of HepG2 Cells Through Regulating Fatty Acid Metabolism
- PMID: 30545108
- PMCID: PMC6321182
- DOI: 10.3390/molecules23123296
Oleiferasaponin A₂, a Novel Saponin from Camellia oleifera Abel. Seeds, Inhibits Lipid Accumulation of HepG2 Cells Through Regulating Fatty Acid Metabolism
Abstract
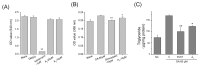
A new triterpenoid saponin, named oleiferasaponin A₂, was isolated and identified from Camellia oleifera defatted seeds. Oleiferasaponin A₂ exhibited anti-hyperlipidemic activity on HepG2 cell lines. Further study of the hypolipidemic mechanism showed that oleiferasaponin A₂ inhibited fatty acid synthesis by significantly down-regulating the expression of SREBP-1c, FAS and FAS protein, while dramatically promoting fatty acid β-oxidation by up-regulating the expression of ACOX-1, CPT-1 and ACOX-1 protein. Our results demonstrate that the oleiferasaponin A₂ possesses potential medicinal value for hyperlipidemia treatment.
Keywords: Camellia oleifera; fatty acid metabolism; hypolipidemic activity; oleiferasaponin A2; triterpenoid saponin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Melanie N., Townsend N., Scarnorough P., Rayner M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014;35:2950–2959. - PubMed
-
- Program L.C. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1985;253:635–636. - PubMed
-
- Zou B., Li C.M., Chen J.Y., Dong X.Q., Zhang Y., Du J. High molecular weight persimmon tannin is a potent hypolipidemic in high-cholesterol diet fed rats. Food Res. Int. 2012;48:970–977. doi: 10.1016/j.foodres.2012.05.024. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

