Mitochondria: multifaceted regulators of aging
- PMID: 30545443
- PMCID: PMC6386233
- DOI: 10.5483/BMBRep.2019.52.1.300
Mitochondria: multifaceted regulators of aging
Abstract
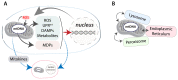
Aging is accompanied by a time-dependent progressive deterioration of multiple factors of the cellular system. The past several decades have witnessed major leaps in our understanding of the biological mechanisms of aging using dietary, genetic, pharmacological, and physical interventions. Metabolic processes, including nutrient sensing pathways and mitochondrial function, have emerged as prominent regulators of aging. Mitochondria have been considered to play a key role largely due to their production of reactive oxygen species (ROS), resulting in DNA damage that accumulates over time and ultimately causes cellular failure. This theory, known as the mitochondrial free radical theory of aging (MFRTA), was favored by the aging field, but increasing inconsistent evidence has led to criticism and rejection of this idea. However, MFRTA should not be hastily rejected in its entirety because we now understand that ROS is not simply an undesired toxic metabolic byproduct, but also an important signaling molecule that is vital to cellular fitness. Notably, mitochondrial function, a term traditionally referred to bioenergetics and apoptosis, has since expanded considerably. It encompasses numerous other key biological processes, including the following: (i) complex metabolic processes, (ii) intracellular and endocrine signaling/communication, and (iii) immunity/inflammation. Here, we will discuss shortcomings of previous concepts regarding mitochondria in aging and their emerging roles based on recent advances. We will also discuss how the mitochondrial genome integrates with major theories on the evolution of aging. [BMB Reports 2019; 52(1): 13-23].
Conflict of interest statement
C.L. is a consultant for and a shareholder of CohBar, Inc. The remaining authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

