Need for comprehensive standardization strategies for marketed Ayurveda formulations
- PMID: 30545738
- PMCID: PMC6314244
- DOI: 10.1016/j.jaim.2018.09.002
Need for comprehensive standardization strategies for marketed Ayurveda formulations
Abstract
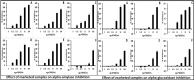
Ayurveda is known for the use of poly-herbal formulations and multi-component therapeutics for the management of health and diseases. Several pharmaceutical companies are manufacturing and marketing different Ayurvedic formulations, prepared as per the classical texts and the regulatory standards. However, on a cursory glance, marked variations are observed amongst the same formulations manufactured by different companies. This raises questions on the quality standards. Drugs or formulations are expected to exert a desired biological activity at particular concentrations of their chemical constituents. The overall aim of drug standardization is to ensure the quality, efficacy and uniformity of the products, in terms of their chemical and biological properties, across the manufactures. In this article, the authors intend to open up a discussion on the need for comprehensive standardization strategies for marketed Ayurveda formulations taking Lodhrasavam (a classical Ayurveda preparation) as an example. Lodhrasavam procured from six reputed Ayurveda drug manufacturers showed significant variations in their sensorial, physico-chemical, chromatographic as well as biological properties. This is a matter of serious concern and need to be addressed effectively to derive better standardization strategies for Ayurvedic formulations.
Keywords: Ayurveda; Formulation standardization; Lodhrasavam; Physico-chemical characters; Sensorial attributes.
Copyright © 2018 Transdisciplinary University, Bangalore and World Ayurveda Foundation. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Chandran U., Mehendale N., Tillu G., Patwardhan B. Network pharmacology of Ayurveda formulation triphala with special reference to anti-cancer property. Comb Chem High Throughput Screen. 2015;18:846–854. - PubMed
-
- Chandran U., Patwardhan B. Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera. J Ethnopharmacol. 2017;197:250–256. - PubMed
-
- Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. - PubMed
LinkOut - more resources
Full Text Sources

