TANGO1 and SEC12 are copackaged with procollagen I to facilitate the generation of large COPII carriers
- PMID: 30545919
- PMCID: PMC6310809
- DOI: 10.1073/pnas.1814810115
TANGO1 and SEC12 are copackaged with procollagen I to facilitate the generation of large COPII carriers
Abstract
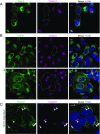
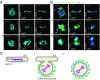
Large coat protein complex II (COPII)-coated vesicles serve to convey the large cargo procollagen I (PC1) from the endoplasmic reticulum (ER). The link between large cargo in the lumen of the ER and modulation of the COPII machinery remains unresolved. TANGO1 is required for PC secretion and interacts with PC and COPII on opposite sides of the ER membrane, but evidence suggests that TANGO1 is retained in the ER, and not included in normal size (<100 nm) COPII vesicles. Here we show that TANGO1 is exported out of the ER in large COPII-coated PC1 carriers, and retrieved back to the ER by the retrograde coat, COPI, mediated by the C-terminal RDEL retrieval sequence of HSP47. TANGO1 is known to target the COPII initiation factor SEC12 to ER exit sites through an interacting protein, cTAGE5. SEC12 is important for the growth of COPII vesicles, but it is not sorted into small budded vesicles. We found both cTAGE5 and SEC12 were exported with TANGO1 in large COPII carriers. In contrast to its exclusion from small transport vesicles, SEC12 was particularly enriched around ER membranes and large COPII carriers that contained PC1. We constructed a split GFP system to recapitulate the targeting of SEC12 to PC1 via the luminal domain of TANGO1. The minimal targeting system enriched SEC12 around PC1 and generated large PC1 carriers. We conclude that TANGO1, cTAGE5, and SEC12 are copacked with PC1 into COPII carriers to increase the size of COPII, thus ensuring the capture of large cargo.
Keywords: COPII; SEC12; TANGO1; collagen; secretion.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Dancourt J, Barlowe C. Protein sorting receptors in the early secretory pathway. Annu Rev Biochem. 2010;79:777–802. - PubMed
-
- Barlowe C, et al. COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
-
- Lee MC, et al. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. - PubMed
-
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

