Structure, activation and dysregulation of fibroblast growth factor receptor kinases: perspectives for clinical targeting
- PMID: 30545934
- PMCID: PMC6299260
- DOI: 10.1042/BST20180004
Structure, activation and dysregulation of fibroblast growth factor receptor kinases: perspectives for clinical targeting
Abstract
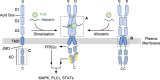
The receptor tyrosine kinase family of fibroblast growth factor receptors (FGFRs) play crucial roles in embryonic development, metabolism, tissue homeostasis and wound repair via stimulation of intracellular signalling cascades. As a consequence of FGFRs' influence on cell growth, proliferation and differentiation, FGFR signalling is frequently dysregulated in a host of human cancers, variously by means of overexpression, somatic point mutations and gene fusion events. Dysregulation of FGFRs is also the underlying cause of many developmental dysplasias such as hypochondroplasia and achondroplasia. Accordingly, FGFRs are attractive pharmaceutical targets, and multiple clinical trials are in progress for the treatment of various FGFR aberrations. To effectively target dysregulated receptors, a structural and mechanistic understanding of FGFR activation and regulation is required. Here, we review some of the key research findings from the last couple of decades and summarise the strategies being explored for therapeutic intervention.
Keywords: drug discovery and design; fibroblast growth factor receptors; receptor tyrosine kinases; structural biology.
© 2018 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

