The trans-Golgi network is a major site for α-secretase processing of amyloid precursor protein in primary neurons
- PMID: 30545942
- PMCID: PMC6364769
- DOI: 10.1074/jbc.RA118.005222
The trans-Golgi network is a major site for α-secretase processing of amyloid precursor protein in primary neurons
Abstract
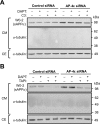
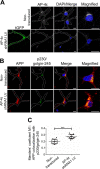
Amyloid precursor protein (APP) is processed along the amyloidogenic pathway by the β-secretase, BACE1, generating β-amyloid (Aβ), or along the nonamyloidogenic pathway by α-secretase, precluding Aβ production. The plasma membrane is considered the major site for α-secretase-mediated APP cleavage, but other cellular locations have not been rigorously investigated. Here, we report that APP is processed by endogenous α-secretase at the trans-Golgi network (TGN) of both transfected HeLa cells and mouse primary neurons. We have previously shown the adaptor protein complex, AP-4, and small G protein ADP-ribosylation factor-like GTPase 5b (Arl5b) are required for efficient post-Golgi transport of APP to endosomes. We found here that AP-4 or Arl5b depletion results in Golgi accumulation of APP and increased secretion of the soluble α-secretase cleavage product sAPPα. Moreover, inhibition of γ-secretase following APP accumulation in the TGN increases the levels of the membrane-bound C-terminal fragments of APP from both α-secretase cleavage (α-CTF, named C83 according to its band size) and BACE1 cleavage (β-CTF/C99). The level of C83 was ∼4 times higher than that of C99, indicating that α-secretase processing is the major pathway and that BACE1 processing is the minor pathway in the TGN. AP-4 silencing in mouse primary neurons also resulted in the accumulation of endogenous APP in the TGN and enhanced α-secretase processing. These findings identify the TGN as a major site for α-secretase processing in HeLa cells and primary neurons and indicate that both APP processing pathways can occur within the TGN compartment along the secretory pathway.
Keywords: adaptor protein; amyloid precursor protein (APP); enzyme processing; membrane trafficking; neurodegenerative disease; protein trafficking (Golgi); secretase; secretion; trans-Golgi network; α-secretase; β-secretase 1 (BACE1).
© 2019 Tan and Gleeson.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
-
- Koo E. H., Squazzo S. L., Selkoe D. J., and Koo C. H. (1996) Trafficking of cell-surface amyloid β-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J. Cell Sci. 109, 991–998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

