Dynamic microtubules drive fibroblast spreading
- PMID: 30545950
- PMCID: PMC6310885
- DOI: 10.1242/bio.038968
Dynamic microtubules drive fibroblast spreading
Abstract
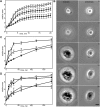
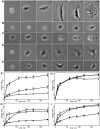
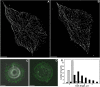
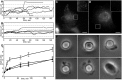
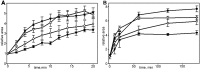
When cells with a mesenchymal type of motility come into contact with an adhesive substrate they adhere and start spreading by the formation of lamellipodia. Using a label-free approach and virtual synchronization approach we analyzed spreading in fibroblasts and cancer cells. In all cell lines spreading is a non-linear process undergoing isotropic or anisotropic modes with first fast (5-20 min) and then slow (30-120 min) phases. In the first 10 min cell area increases 2-4 times, while the absolute rate of initial spreading decreases 2-8 times. Fast spreading depends on actin polymerization and dynamic microtubules. Inhibition of microtubule growth was sufficient for a slowdown of initial spreading. Inhibition of myosin II in the presence of stable microtubules restored fast spreading. Inhibition of actin polymerization or complete depolymerization of microtubules slowed down fast spreading. However, in these cases inhibition of myosin II only partially restored spreading kinetics. We conclude that rapid growth of microtubules towards cell margins at the first stage of cell spreading temporarily inhibits phosphorylation of myosin II and is essential for the fast isotropic spreading. Comparison of the fibroblasts with cancer cells shows that fast spreading in different cell types shares similar kinetics and mechanisms, and strongly depends on dynamic microtubules.
Keywords: Cell spreading; Cytoskeleton; Microtubule dynamics; Myosin II.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Bardsley W. G. and Aplin J. D. (1983). Kinetic analysis of cell spreading. I. Theory and modelling of curves. J. Cell Sci. 61, 365-373. - PubMed
-
- Bereiter-Hahn J., Luck M., Miebach T., Stelzer H. K. and Voth M. (1990). Spreading of trypsinized cells: cytoskeletal dynamics and energy requirements. J. Cell Sci. 96, 171-188. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

