Risk stratification and medical therapy of pulmonary arterial hypertension
- PMID: 30545971
- PMCID: PMC6351343
- DOI: 10.1183/13993003.01889-2018
Risk stratification and medical therapy of pulmonary arterial hypertension
Abstract
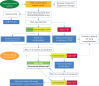
Pulmonary arterial hypertension (PAH) remains a severe clinical condition despite the availability over the past 15 years of multiple drugs interfering with the endothelin, nitric oxide and prostacyclin pathways. The recent progress observed in medical therapy of PAH is not, therefore, related to the discovery of new pathways, but to the development of new strategies for combination therapy and on escalation of treatments based on systematic assessment of clinical response. The current treatment strategy is based on the severity of the newly diagnosed PAH patient as assessed by a multiparametric risk stratification approach. Clinical, exercise, right ventricular function and haemodynamic parameters are combined to define a low-, intermediate- or high-risk status according to the expected 1-year mortality. The current treatment algorithm provides the most appropriate initial strategy, including monotherapy, or double or triple combination therapy. Further treatment escalation is required in case low-risk status is not achieved in planned follow-up assessments. Lung transplantation may be required in most advanced cases on maximal medical therapy.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: N. Galiè reports grants and personal fees from Actelion, Bayer, GSK and Pfizer, and personal fees from MSD, outside the submitted work. Conflict of interest: R.N. Channick reports grants and personal fees from Actelion and Bayer, and personal fees from Arena, outside the submitted work. Conflict of interest: R.P. Frantz reports steering committee membership and research funding from Actelion, data and safety monitoring board and adjudication committee membership for United Therapeutics, and advisory board work for Abbott and Arena, outside the submitted work. Conflict of interest: E. Grünig reports grants and personal fees from Actelion and Bayer/MSD, grants from GSK, and personal fees from Bial, OrPhaSwiss GmbH and Medscape, outside the submitted work. Conflict of interest: Z.C. Jing reports grants from the Chinese Academy of Medical Sciences, Beijing Natural Science Foundation and National Natural Science Foundation of China, during the conduct of the study; and personal fees from Actelion Pharmaceuticals, Bayer Healthcare Pharmaceuticals, GSK, Pfizer and United Therapeutics, outside the submitted work. Conflict of interest: O. Moiseeva has nothing to disclose. Conflict of interest: I.R. Preston reports grants and personal fees for consultancy from Actelion, Gilead and United Therapeutics, grants from Bayer, and personal fees for adjudication committee membership from Pfizer, during the conduct of the study; and grants and personal fees for consultancy from Acceleron, Liquidia and Arena, outside the submitted work. Conflict of interest: T. Pulido reports research grants from Actelion, Lilly, Reata Pharmaceuticals and Bayer, personal fees for advisory board membership, speaking and lectures from Actelion, personal fees for lectures from Bayer, personal fees for advisory board membership from GSK, personal fees for advisory board membership and lectures from Pfizer, outside the submitted work. Conflict of interest: Z. Safdar reports speaker bureau, consultation and institutional grants for clinical trials from Actelion Pharmaceutical, United Therapeutics Gilead Pharmaceuticals, Genetech, Boehringer Ingelheim and Bayer Pharmaceuticals, outside the submitted work. Conflict of interest: Y. Tamura reports grants from Nippon Shinyaku Co., Ltd, and grants and personal fees from Actelion Pharmaceuticals Japan Ltd, during the conduct of the study. Conflict of interest: V.V. McLaughlin reports grants and personal fees from Actelion, Acceleron, Arena and Bayer, grants from Gilead and Sonovie, and personal fees from Caremark and United Therapeutics, during the conduct of the study.
Figures

Comment in
References
-
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016; 37: 67–119. - PubMed
-
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. - PubMed
-
- Galiè N, Seeger W, Naeije R, et al. Comparative analysis of clinical trials and evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43: 12 Suppl. S, S81–S88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
