Quantity and Reporting Quality of Kidney Research
- PMID: 30545982
- PMCID: PMC6317598
- DOI: 10.1681/ASN.2018050515
Quantity and Reporting Quality of Kidney Research
Abstract
Background: In 2004, researchers reported that the number of nephrology clinical trials was low and that the reporting quality of such trials was suboptimal. Furthermore, the number or quality of preclinical kidney-related studies has not been systematically evaluated.
Methods: We performed a systematic review of randomized clinical trials published in 1966-2017 (listed in the Cochrane Library) and preclinical studies published in 1945-2017 (listed in PubMed). For reporting quality analysis, we evaluated the final main paper of 118 clinical trial reports and 135 preclinical studies published in leading journals in 1996, 2006, and 2016 on the basis of criteria from the widely used CONSORT and ARRIVE guidelines.
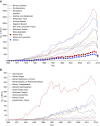
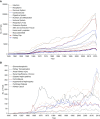
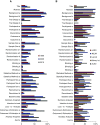
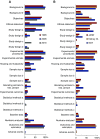
Results: The annual number of reports of clinical kidney-related trials more than doubled between 2004 and 2014 along with reports in other medical disciplines. Hypertension remains the dominant focus of study, but ongoing trials also center on CKD, ESRD, and AKI. The reporting quality analysis revealed improvements, but deficits in reporting of clinical trial design, mode of randomization, and intention-to-treat analysis remain. Annual numbers of kidney-related preclinical studies remained low between 1945 and 2017 compared with other disciplines. Reporting quality analysis of preclinical studies revealed substantial reporting deficits across all leading journals, with little improvement over the last 20 years, especially for group size calculations, defining primary versus secondary outcomes, and blinded analysis.
Conclusions: Nephrology studies keep increasing in number but still lag behind other medical disciplines, and the quality of data reporting in kidney research can be further improved.
Keywords: clinical trial; kidney; kidney disease.
Copyright © 2019 by the American Society of Nephrology.
Figures

Comment in
-
The Quality of Reporting of Kidney Research: A Challenge to JASN.J Am Soc Nephrol. 2019 Jan;30(1):1-2. doi: 10.1681/ASN.2018111132. Epub 2018 Dec 13. J Am Soc Nephrol. 2019. PMID: 30545983 Free PMC article. No abstract available.
-
Compromising Outcomes.J Am Soc Nephrol. 2019 Jul;30(7):1147-1150. doi: 10.1681/ASN.2019010057. Epub 2019 Jun 17. J Am Soc Nephrol. 2019. PMID: 31208985 Free PMC article. No abstract available.
References
-
- Strippoli GF, Craig JC, Schena FP: The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 15: 411–419, 2004 - PubMed
-
- CONSORT. Available at: http://www.consort-statement.org/. Accessed September 9, 2017.
-
- International Committee of Medical Journal Editors: Clinical Trials. Available at: http://www.icmje.org/recommendations/browse/publishing-and-editorial-iss.... Accessed September 22, 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

