Augmentation of Atrazine biodegradation by two Bacilli immobilized on α-Fe2O3 magnetic nanoparticles
- PMID: 30546039
- PMCID: PMC6292855
- DOI: 10.1038/s41598-018-36296-1
Augmentation of Atrazine biodegradation by two Bacilli immobilized on α-Fe2O3 magnetic nanoparticles
Abstract
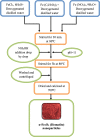
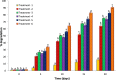
In this study, a novel immobilizing carrier with α-Fe2O3 magnetic nanoparticles was developed and used for immobilization of atrazine-degrading bacterial isolates of Bacillus spp. Since the free cells of microorganisms generally not succeed to degrade pollutants; thus, extra treatments are alluring to make strides biodegradation. Scanning electron microscope (SEM) images appeared that after immobilization the bacterial cells were totally retained and entirely distributed on the surface of α-Fe2O3 magnetic nanoparticles. The performance of α-Fe2O3 immobilized cells in atrazine (ATZ) degradation was compared with the free cells, which was about 90.56% in 20 days. Experimental results exhibited that ATZ could be degraded at a broad range of physicochemical parameters viz. pH (4.0 to 9.0), temperature (20 to 45 °C), ATZ concentration (50 to 300 mg L-1) and agitation speed (50 to 300 rpm), which underlines that α-Fe2O3 immobilized cells could tolerate a higher range of ATZ concentration as compared to free cells. This research demonstrated that α-Fe2O3 could be applied as a potential carrier in cell immobilization and biodegradation of ATZ herbicide with greater efficiency.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Alginate immobilized enrichment culture for atrazine degradation in soil and water system.J Environ Sci Health B. 2017 Apr 3;52(4):229-236. doi: 10.1080/03601234.2016.1270680. Epub 2017 Jan 12. J Environ Sci Health B. 2017. PMID: 28080203
-
Sugarcane bagasse as support for immobilization of Bacillus pumilus HZ-2 and its use in bioremediation of mesotrione-contaminated soils.Appl Microbiol Biotechnol. 2015 Dec;99(24):10839-51. doi: 10.1007/s00253-015-6935-0. Epub 2015 Sep 4. Appl Microbiol Biotechnol. 2015. PMID: 26337896
-
Evaluating phytochemical and microbial contributions to atrazine degradation.J Environ Manage. 2022 Nov 1;321:115840. doi: 10.1016/j.jenvman.2022.115840. Epub 2022 Aug 19. J Environ Manage. 2022. PMID: 35994960
-
Magnetic immobilization of bacteria using iron oxide nanoparticles.Biotechnol Lett. 2018 Feb;40(2):237-248. doi: 10.1007/s10529-017-2477-0. Epub 2017 Nov 27. Biotechnol Lett. 2018. PMID: 29181762 Review.
-
Evolution of atrazine-degrading capabilities in the environment.Appl Microbiol Biotechnol. 2012 Dec;96(5):1175-89. doi: 10.1007/s00253-012-4495-0. Epub 2012 Oct 18. Appl Microbiol Biotechnol. 2012. PMID: 23076592 Review.
Cited by
-
Optimization studies on biodegradation of atrazine by Bacillus badius ABP6 strain using response surface methodology.Biotechnol Rep (Amst). 2020 Apr 28;26:e00459. doi: 10.1016/j.btre.2020.e00459. eCollection 2020 Jun. Biotechnol Rep (Amst). 2020. PMID: 32395437 Free PMC article.
-
Biodegradation of Alprazolam in Pharmaceutical Wastewater Using Mesoporous Nanoparticles-Adhered Pseudomonas stutzeri.Molecules. 2021 Dec 31;27(1):237. doi: 10.3390/molecules27010237. Molecules. 2021. PMID: 35011469 Free PMC article.
-
Biocatalytic System Made of 3D Chitin, Silica Nanopowder and Horseradish Peroxidase for the Removal of 17α-Ethinylestradiol: Determination of Process Efficiency and Degradation Mechanism.Molecules. 2022 Feb 17;27(4):1354. doi: 10.3390/molecules27041354. Molecules. 2022. PMID: 35209143 Free PMC article.
-
Immobilization of Biomass Materials for Removal of Refractory Organic Pollutants from Wastewater.Int J Environ Res Public Health. 2022 Oct 24;19(21):13830. doi: 10.3390/ijerph192113830. Int J Environ Res Public Health. 2022. PMID: 36360710 Free PMC article. Review.
-
Mechanistic and recent updates in nano-bioremediation for developing green technology to alleviate agricultural contaminants.Int J Environ Sci Technol (Tehran). 2022 Sep 29:1-26. doi: 10.1007/s13762-022-04560-7. Online ahead of print. Int J Environ Sci Technol (Tehran). 2022. PMID: 36196301 Free PMC article. Review.
References
-
- Ghosh PK, Philip L. Environmental significance of atrazine in aqueous systems and its removal by biological processes: an overview. Global Nest journal. 2006;8:71–90.
-
- Kaushik G, Satya S, Naik SN. Food processing a tool to pesticide residue dissipation - A review. Food Research International. 2009;42:26–40. doi: 10.1016/j.foodres.2008.09.009. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

