Intron-containing RNA from the HIV-1 provirus activates type I interferon and inflammatory cytokines
- PMID: 30546110
- PMCID: PMC6294009
- DOI: 10.1038/s41467-018-07753-2
Intron-containing RNA from the HIV-1 provirus activates type I interferon and inflammatory cytokines
Abstract
HIV-1-infected people who take drugs that suppress viremia to undetectable levels are protected from developing AIDS. Nonetheless, HIV-1 establishes proviruses in long-lived CD4+ memory T cells, and perhaps other cell types, that preclude elimination of the virus even after years of continuous antiviral therapy. Here we show that the HIV-1 provirus activates innate immune signaling in isolated dendritic cells, macrophages, and CD4+ T cells. Immune activation requires transcription from the HIV-1 provirus and expression of CRM1-dependent, Rev-dependent, RRE-containing, unspliced HIV-1 RNA. If rev is provided in trans, all HIV-1 coding sequences are dispensable for activation except those cis-acting sequences required for replication or splicing. Our results indicate that the complex, post-transcriptional regulation intrinsic to HIV-1 RNA is detected by the innate immune system as a danger signal, and that drugs which disrupt HIV-1 transcription or HIV-1 RNA metabolism would add qualitative benefit to current antiviral drug regimens.
Conflict of interest statement
The authors declare no competing interests.
Figures
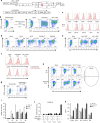

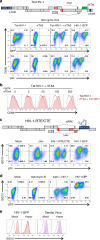
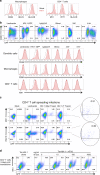
Similar articles
-
A lentiviral vector that activates latent human immunodeficiency virus-1 proviruses by the overexpression of tat and that kills the infected cells.Hum Gene Ther. 2009 Nov;20(11):1259-68. doi: 10.1089/hum.2009.059. Hum Gene Ther. 2009. PMID: 19604078
-
IFIH1 (MDA5) is required for innate immune detection of intron-containing RNA expressed from the HIV-1 provirus.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2404349121. doi: 10.1073/pnas.2404349121. Epub 2024 Jul 10. Proc Natl Acad Sci U S A. 2024. PMID: 38985764 Free PMC article.
-
Mathematical model of the Tat-Rev regulation of HIV-1 replication in an activated cell predicts the existence of oscillatory dynamics in the synthesis of viral components.BMC Genomics. 2014;15 Suppl 12(Suppl 12):S1. doi: 10.1186/1471-2164-15-S12-S1. Epub 2014 Dec 19. BMC Genomics. 2014. PMID: 25564443 Free PMC article.
-
Inhibition of HIV-1 replication by targeting the Rev protein.Leukemia. 1997 Apr;11 Suppl 3:134-7. Leukemia. 1997. PMID: 9209321 Review.
-
HIV Persistence in Adipose Tissue Reservoirs.Curr HIV/AIDS Rep. 2018 Feb;15(1):60-71. doi: 10.1007/s11904-018-0378-z. Curr HIV/AIDS Rep. 2018. PMID: 29423731 Free PMC article. Review.
Cited by
-
Sex differences in HIV-1 persistence and the implications for a cure.Front Glob Womens Health. 2022 Sep 23;3:942345. doi: 10.3389/fgwh.2022.942345. eCollection 2022. Front Glob Womens Health. 2022. PMID: 36212905 Free PMC article. Review.
-
Indels allow antiviral proteins to evolve functional novelty inaccessible by missense mutations.Cell Genom. 2025 Jun 11;5(6):100818. doi: 10.1016/j.xgen.2025.100818. Epub 2025 Mar 25. Cell Genom. 2025. PMID: 40139185 Free PMC article.
-
The intestinal interferon system and specialized enterocytes as putative drivers of HIV latency.Front Immunol. 2025 May 14;16:1589752. doi: 10.3389/fimmu.2025.1589752. eCollection 2025. Front Immunol. 2025. PMID: 40438119 Free PMC article. Review.
-
IFIH1 (MDA5) is required for innate immune detection of intron-containing RNA expressed from the HIV-1 provirus.bioRxiv [Preprint]. 2023 Dec 12:2023.11.17.567619. doi: 10.1101/2023.11.17.567619. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2404349121. doi: 10.1073/pnas.2404349121. PMID: 38014177 Free PMC article. Updated. Preprint.
-
The complex life of the HIV-1 full-length RNA.Nat Rev Microbiol. 2024 Jun;22(6):325. doi: 10.1038/s41579-024-01049-7. Nat Rev Microbiol. 2024. PMID: 38658789 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

