A Cell Atlas for the Mouse Brain
- PMID: 30546301
- PMCID: PMC6280067
- DOI: 10.3389/fninf.2018.00084
A Cell Atlas for the Mouse Brain
Erratum in
-
Corrigendum: A Cell Atlas for the Mouse Brain.Front Neuroinform. 2019 Feb 19;13:7. doi: 10.3389/fninf.2019.00007. eCollection 2019. Front Neuroinform. 2019. PMID: 30837861 Free PMC article.
Abstract
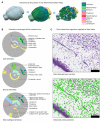
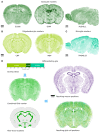
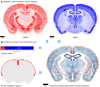
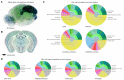
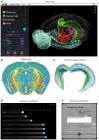
Despite vast numbers of studies of stained cells in the mouse brain, no current brain atlas provides region-by-region neuron counts. In fact, neuron numbers are only available for about 4% of brain of regions and estimates often vary by as much as 3-fold. Here we provide a first 3D cell atlas for the whole mouse brain, showing cell positions constructed algorithmically from whole brain Nissl and gene expression stains, and compared against values from the literature. The atlas provides the densities and positions of all excitatory and inhibitory neurons, astrocytes, oligodendrocytes, and microglia in each of the 737 brain regions defined in the AMBA. The atlas is dynamic, allowing comparison with previously reported numbers, addition of cell types, and improvement of estimates as new data is integrated. The atlas also provides insights into cellular organization only possible at this whole brain scale, and is publicly available.
Keywords: Allen brain atlas; cell numbers; glia; mouse brain; neurons.
Figures



References
-
- Cahoy J. D., Emery B., Kaushal A., Foo L. C., Zamanian J. L., Christopherson K. S., et al. . (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278. 10.1523/JNEUROSCI.4178-07.2008 - DOI - PMC - PubMed
-
- Dong H. W. (2008). The Allen Reference Atlas: A Digital Color Brain Atlas of the C57Bl/6J Male Mouse. Hoboken, NJ: John Wiley & Sons Inc.
-
- Förster J. (2008). Quantitative Morphological Analysis of the Neostriatum and the Cerebellum of Tenascin-C Deficient Mice. Doctoral Dissertation, University of Hamburg-Eppendorf; Hamburg, Germany.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

