Bone Marrow Fat and Hematopoiesis
- PMID: 30546345
- PMCID: PMC6280186
- DOI: 10.3389/fendo.2018.00694
Bone Marrow Fat and Hematopoiesis
Abstract
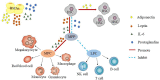
Bone marrow fat cells comprise the largest population of cells in the bone marrow cavity, a characteristic that has attracted the attention of scholars from different disciplines. The perception that bone marrow adipocytes are "inert space fillers" has been broken, and currently, bone marrow fat is unanimously considered to be the third largest fat depot, after subcutaneous fat and visceral fat. Bone marrow fat (BMF) acts as a metabolically active organ and plays an active role in energy storage, endocrine function, bone metabolism, and the bone metastasis of tumors. Bone marrow adipocytes (BMAs), as a component of the bone marrow microenvironment, influence hematopoiesis through direct contact with cells and the secretion of adipocyte-derived factors. They also influence the progression of hematologic diseases such as leukemia, multiple myeloma, and aplastic anemia, and may be a novel target when exploring treatments for related diseases in the future. Based on currently available data, this review describes the role of BMF in hematopoiesis as well as in the development of hematologic diseases.
Keywords: aplastic anemia; bone marrow fat; hematopoiesis; leukemia; multiple myeloma.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

