Lutzomyia longipalpis Saliva Induces Heme Oxygenase-1 Expression at Bite Sites
- PMID: 30546363
- PMCID: PMC6279893
- DOI: 10.3389/fimmu.2018.02779
Lutzomyia longipalpis Saliva Induces Heme Oxygenase-1 Expression at Bite Sites
Abstract
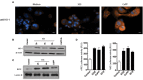
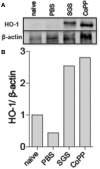
Sand flies bite mammalian hosts to obtain a blood meal, driving changes in the host inflammatory response that support the establishment of Leishmania infection. This effect is partially attributed to components of sand fly saliva, which are able to recruit and activate leukocytes. Our group has shown that heme oxygenase-1 (HO-1) favors Leishmania survival in infected cells by reducing inflammatory responses. Here, we show that exposure to sand fly bites is associated with induction of HO-1 in vivo. Histopathological analyses of skin specimens from human volunteers experimentally exposed to sand fly bites revealed that HO-1 and Nrf2 are produced at bite sites in the skin. These results were recapitulated in mice ears injected with a salivary gland sonicate (SGS) or exposed to sand fly bites, indicating that vector saliva may be a key factor in triggering HO-1 expression. Resident skin macrophages were the main source HO-1 at 24-48 h after bites. Additionally, assays in vivo after bites and in vitro after stimulation with saliva both demonstrated that HO-1 production by macrophages was Nrf2-dependent. Collectively, our data demonstrates that vector saliva induces early HO-1 production at the bite sites, representing a major event associated with establishment of naturally-transmitted Leishmania infections.
Keywords: Lutzomyia longipalpis; Nrf2; heme oxygenase-1; macrophages; saliva; sand fly bite; skin.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

