Efficacy of several biological therapies for treating moderate to severe psoriasis: A network meta-analysis
- PMID: 30546410
- PMCID: PMC6257037
- DOI: 10.3892/etm.2018.6859
Efficacy of several biological therapies for treating moderate to severe psoriasis: A network meta-analysis
Abstract
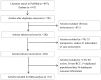
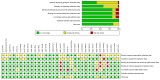
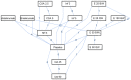
The aim of the present meta-analysis was to systematically assess the efficacy of the various treatments available for moderate to severe psoriasis. PubMed and Embase databases were systematically searched to select relevant studies up to February 2015. Odds ratios (ORs) and their 95% confidence intervals (CIs) were used as effect estimates. In addition, the Psoriasis Area and Severity Index (PASI) 50, PASI 75 and PASI 90 responses for the therapies were systematically assessed. A total of 33 randomized controlled trials were included in the present study. For the PASI 75 response rate, infliximab (5 mg) may be the most effective option for the treatment of moderate to severe psoriasis. Furthermore, the pooled results of the PASI 50 response rate demonstrated that infliximab (5 mg) and ustekinumab (90 mg) may be superior to other drugs for treating moderate to severe psoriasis. For the PASI 90 response rate, infliximab (5 mg), ustekinumab (90 mg) and briakinumab (weeks 0 and 4, 200 mg; week 8, 100 mg) exhibited improved results compared with other treatments. In conclusion, infliximab (5 mg) may be a superior option to treat moderate to severe psoriasis due to the relatively high PASI scores. However, despite the high PASI 90 responses, further studies are required to identify the efficacy of ustekinumab (90 mg) and briakinumab.
Keywords: Psoriasis Area and Severity Index; biological therapies; network meta-analysis; psoriasis.
Figures
References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team, corp-author. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. - DOI - PubMed
-
- Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. - PubMed
-
- Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E, Gladman D, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: Results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–1236. doi: 10.1002/art.20967. - DOI - PubMed
-
- Asahina A, Nakagawa H, Etoh T, Ohtsuki M Adalimumab M04-688 Study Group, corp-author. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: Efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. 2010;37:299–310. doi: 10.1111/j.1346-8138.2009.00748.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
