Relationship between the genetic expression of WTAP and bladder cancer and patient prognosis
- PMID: 30546429
- PMCID: PMC6256415
- DOI: 10.3892/ol.2018.9554
Relationship between the genetic expression of WTAP and bladder cancer and patient prognosis
Abstract
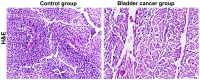
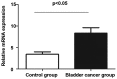
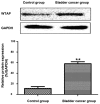
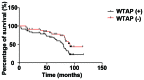
The relationship between the gene expression of Wilms tumor 1-associated protein (WTAP) and bladder cancer was investigated to study the effect of its expression on patient prognosis. Sixty-two fresh specimens of bladder transitional cell cancer tissues were collected as the bladder cancer group, while 20 normal bladder mucosa specimens comprised the control group. Hematoxylin and eosin staining was conducted to detect the pathological differences between the groups and the immunohistochemistry was used to test the expression levels of WTAP in the tissues. Reverse transcription-polymerase chain reaction (RT-PCR) was used to detect the mRNA expression levels of WTAP. Moreover, western blot analysis was used to examine the WTAP expression levels. At the same time, Cox regression multi-factor survival analysis was conducted for the related factors to the prognoses of bladder cancer patients. The structures of cells in the bladder cancer group were destroyed as was evident by the shrunken cell nuclei, while the tissues in the control group were intact. WTAP expression in the bladder cancer group was significantly increased compared with that in the control group. A small number of mRNAs and proteins were higher in the bladder cancer group than that in the control group. The differences in WTAP expression between the bladder cancer and control groups were statistically significant (P<0.05). There were obvious differences in the postoperative recurrence risk between the patients with a negative WTAP protein expression and those with a positive one (P<0.05). In conclusion, WTAP may play an important role in the occurrence and development of bladder cancer and can be considered as a potential target for bladder cancer treatment, providing a new basis for clinical diagnoses.
Keywords: WTAP; bladder cancer; relationship with prognosis.
Figures

References
-
- Fiebig HH, Dengler AW, Roth T. Human tumor xenografts: predictivity, characterization and discovery of new anticancer agents. In: Fiebig HH, Burger AM, editors. Contributions to Oncology. Relevance of Tumor Models for Anticancer Drug Development. Vol. 54. Contr Oncol Basel; Karger: 1999. pp. 29–50.
LinkOut - more resources
Full Text Sources
