Synthesis of indole-cycloalkyl[ b]pyridine hybrids via a four-component six-step tandem process
- PMID: 30546474
- PMCID: PMC6278771
- DOI: 10.3762/bjoc.14.269
Synthesis of indole-cycloalkyl[ b]pyridine hybrids via a four-component six-step tandem process
Abstract
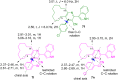
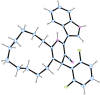
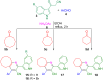
The one-pot four-component reaction of 3-(1H-indol-3-yl)-3-oxopropanenitriles, aromatic aldehydes, cycloalkanones and ammonium acetate occurred via a six-step tandem Knoevenagel condensation-nucleophilic addition to carbonyl-Michael addition-N-cyclization-elimination-air oxidation sequence to afford structurally intriguing indole-cycloalkyl[b]pyridine-3-carbonitrile hybrid heterocycles in excellent yields.
Keywords: 3-(1H-indol-3-yl)-3-oxopropanenitrile; cycloalkyl[b]pyridine-3-carbonitrile; cyclododecanone; tandem reaction.
Figures

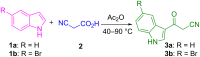



References
LinkOut - more resources
Full Text Sources
