Copper(I)-catalyzed tandem reaction: synthesis of 1,4-disubstituted 1,2,3-triazoles from alkyl diacyl peroxides, azidotrimethylsilane, and alkynes
- PMID: 30546475
- PMCID: PMC6278767
- DOI: 10.3762/bjoc.14.270
Copper(I)-catalyzed tandem reaction: synthesis of 1,4-disubstituted 1,2,3-triazoles from alkyl diacyl peroxides, azidotrimethylsilane, and alkynes
Abstract
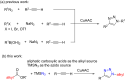
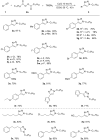
A copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction for the synthesis of 1,4-disubstituted 1,2,3-triazoles from alkyl diacyl peroxides, azidotrimethylsilane, and terminal alkynes is reported. The alkyl carboxylic acids is for the first time being used as the alkyl azide precursors in the form of alkyl diacyl peroxides. This method avoids the necessity to handle organic azides, as they are generated in situ, making this protocol operationally simple. The Cu(I) catalyst not only participates in the alkyl diacyl peroxides decomposition to afford alkyl azides but also catalyzes the subsequent CuAAC reaction to produce the 1,2,3-triazoles.
Keywords: 1,2,3-triazoles; alkyl diacyl peroxides; azidotrimethylsilane; click reaction; copper catalysis; radical.
Figures



Similar articles
-
Quick and highly efficient copper-catalyzed cycloaddition of organic azides with terminal alkynes.Org Biomol Chem. 2012 Jan 14;10(2):229-31. doi: 10.1039/c1ob06190a. Epub 2011 Oct 24. Org Biomol Chem. 2012. PMID: 22024945
-
Copper-Catalyzed Azide-Alkyne Cycloaddition of Hydrazoic Acid Formed In Situ from Sodium Azide Affords 4-Monosubstituted-1,2,3-Triazoles.J Org Chem. 2022 Mar 18;87(6):4018-4028. doi: 10.1021/acs.joc.1c02775. Epub 2022 Feb 11. J Org Chem. 2022. PMID: 35148087 Free PMC article.
-
On-DNA-1,2,3-Triazole Formation via Click Reaction.Methods Mol Biol. 2022;2541:39-43. doi: 10.1007/978-1-0716-2545-3_6. Methods Mol Biol. 2022. PMID: 36083541
-
In-situ Generated and Premade 1-Copper(I) Alkynes in Cycloadditions.Chem Rec. 2017 Dec;17(12):1231-1248. doi: 10.1002/tcr.201700011. Epub 2017 Jun 22. Chem Rec. 2017. PMID: 28639363 Review.
-
Recent Advances in Recoverable Systems for the Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction (CuAAC).Molecules. 2016 Sep 5;21(9):1174. doi: 10.3390/molecules21091174. Molecules. 2016. PMID: 27607998 Free PMC article. Review.
Cited by
-
In situ activation of therapeutics through bioorthogonal catalysis.Adv Drug Deliv Rev. 2021 Sep;176:113893. doi: 10.1016/j.addr.2021.113893. Epub 2021 Jul 29. Adv Drug Deliv Rev. 2021. PMID: 34333074 Free PMC article. Review.
-
Development of Essential Oil Delivery Systems by 'Click Chemistry' Methods: Possible Ways to Manage Duchenne Muscular Dystrophy.Materials (Basel). 2023 Oct 2;16(19):6537. doi: 10.3390/ma16196537. Materials (Basel). 2023. PMID: 37834674 Free PMC article. Review.
References
-
- Nair D P, Podgórski M, Chatani S, Gong T, Xi W, Fenoli C R, Bowman C N. Chem Mater. 2014;26(1):724–744. doi: 10.1021/cm402180t. - DOI
LinkOut - more resources
Full Text Sources
