Late adult-onset adrenomyeloneuropathy evolving with atypical severe frontal lobe syndrome: Importance of neuroimaging
- PMID: 30546814
- PMCID: PMC6282458
- DOI: 10.1016/j.radcr.2018.11.007
Late adult-onset adrenomyeloneuropathy evolving with atypical severe frontal lobe syndrome: Importance of neuroimaging
Abstract
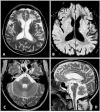
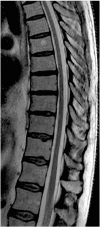
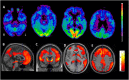
X-linked adrenoleukodystrophy (X-ALD) is a rare inherited metabolic disease affecting the nervous system and the adrenal glands. It is caused by a mutation of the ABCD1 gene, resulting in the impaired degradation of very long-chain fatty acids and their subsequent accumulation in several organs and tissues. X-ALD is notable for its high phenotypical variability, that includes isolated adrenocortical insufficiency, slowly progressive myelopathy with paraparesis, ataxia, and peripheral neuropathy to severe childhood cerebral forms. Here, we describe the case of an X-ALD patient with a p.Gly343Val mutation in ABCD1 gene, who presented in adulthood with a spinal syndrome of mild severity, and later developed a progressive cognitive and behavioral syndrome. Our patient showed a striking correlation between clinical phenotype and neuroimaging, including a brain fluoro-2-deoxy-d-glucose positron emission tomography that displayed an atypical cerebral glucose metabolism.
Keywords: Brain FDG-PET; Cortical and subcortical atrophy; Frontal lobe dysfunction; Missense mutation; X-linked adrenoleukodystrophy (X-ALD).
Figures
Similar articles
-
Broadening the Spectrum of Adulthood X-Linked Adrenoleukodystrophy: A Report of Two Atypical Cases.Front Neurol. 2019 Feb 6;10:70. doi: 10.3389/fneur.2019.00070. eCollection 2019. Front Neurol. 2019. PMID: 30787906 Free PMC article.
-
X-linked adrenoleukodystrophy in a chimpanzee due to an ABCD1 mutation reported in multiple unrelated humans.Mol Genet Metab. 2017 Nov;122(3):130-133. doi: 10.1016/j.ymgme.2017.08.012. Epub 2017 Sep 1. Mol Genet Metab. 2017. PMID: 28919002
-
[X-linked adrenoleukodystrophy].Ann Endocrinol (Paris). 2007 Dec;68(6):403-11. doi: 10.1016/j.ando.2007.04.002. Epub 2007 May 29. Ann Endocrinol (Paris). 2007. PMID: 17532287 Review. French.
-
Adult onset cerebral form of X-linked adrenoleukodystrophy with dementia of frontal lobe type with new L160P mutation in ABCD1 gene.J Neurol Sci. 2007 Dec 15;263(1-2):149-53. doi: 10.1016/j.jns.2007.01.082. Epub 2007 Jul 26. J Neurol Sci. 2007. PMID: 17662307
-
Endocrine Dysfunction in X-Linked Adrenoleukodystrophy.Endocrinol Metab Clin North Am. 2016 Jun;45(2):295-309. doi: 10.1016/j.ecl.2016.01.003. Epub 2016 Apr 13. Endocrinol Metab Clin North Am. 2016. PMID: 27241966 Review.
Cited by
-
A novel ABCD1 G1202A mutation in a Chinese patient with pure adrenomyeloneuropathy and literature review.Genes Dis. 2020 Jan 28;8(5):709-714. doi: 10.1016/j.gendis.2020.01.009. eCollection 2021 Sep. Genes Dis. 2020. PMID: 34291142 Free PMC article.
-
Initial frontal lobe involvement in adult cerebral X-linked adrenoleukodystrophy.Acta Neurol Belg. 2023 Dec;123(6):2259-2268. doi: 10.1007/s13760-023-02295-x. Epub 2023 May 29. Acta Neurol Belg. 2023. PMID: 37247117
-
Burden of illness and mortality in men with Adrenomyeloneuropathy: a retrospective cohort study.Orphanet J Rare Dis. 2024 Jul 17;19(1):270. doi: 10.1186/s13023-024-03276-w. Orphanet J Rare Dis. 2024. PMID: 39020416 Free PMC article.
References
-
- Berger J., Gartner J. X-linked adrenoleukodystrophy: clinical, biochemical and pathogenetic aspects. Biochim Biophys Acta. 2006;1763:1721–1732. - PubMed
-
- Moser H.W., Mahmood A., Raymond G.V. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3:140–151. - PubMed
-
- van Roermund C.W., Visser W.F., Ijlst L., van Cruchten A., Boek M., Kulik W. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–4208. - PubMed
-
- Wiesinger C., Kunze M., Regelsberger G., Forss-Petter S., Berger J. Impaired very long-chain acyl-CoA beta-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction. J Biol Chem. 2013;288:19269–19279. doi: 10.1074/jbc.M112.445445. doi: - DOI - PMC - PubMed
-
- Cotrufo R., Melone M.A., Monsurro M.R., Di Iorio G., Carella C., Moser H.W. Phenotype heterogeneity among hemizygotes in a family biochemically screened for adrenoleukodystrophy. Am J Med Genet. 1987;26:833–838. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

