Pharmacokinetics of 10-gingerol and 6-shogaol in the plasma of healthy subjects treated with red ginger (Zingiber officinale var. Rubrum) suspension
- PMID: 30546874
- PMCID: PMC6256190
- DOI: 10.3892/br.2018.1163
Pharmacokinetics of 10-gingerol and 6-shogaol in the plasma of healthy subjects treated with red ginger (Zingiber officinale var. Rubrum) suspension
Abstract
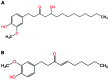
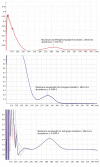
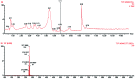
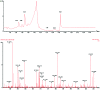
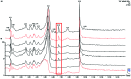
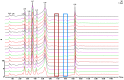
Red ginger (Zingiber officinale var. Rubrum) is among the most widely consumed medicinal herbs in Indonesia. Ginger rhizome contains phenol compounds including gingerol and shogaol. 10-gingerol has been reported to exhibit the greatest anti-inflammatory and anti-oxidant activities compared with those of other gingerols. Pharmacokinetic studies on ginger have been reported, but there is a lack of such study on red ginger. The present work studied the pharmacokinetics of 10-gingerol and 6-shogaol in the plasma of healthy subjects treated with a single dose of red ginger suspension. Healthy subjects (n=19) were given a single dose of red ginger suspension (2 g/15 ml), and blood samples were taken at baseline (0 min), 30, 60, 90, 120, and 180 min. Analysis of 10-gingerol and 6-shogaol was performed by dissolving 200 µl of the subjects' plasma in 800 µl acetonitrile. The mixture was vortexed and centrifuged at 20,440 × g for 15 min at room temperature. The supernatant was filtered using Millipore membrane (pore size 0.2 µm) and injected into an RP-C18 column for liquid chromatography-mass spectrometry. A mixture of 0.1% (v/v) formic acid in water and acetonitrile (38:62) was used as the mobile phase. The maximum plasma concentration (Cmax) and time to reach Cmax of 10-gingerol and 6-shogaol were 160.49 ng/ml (38 min) and 453.40 ng/ml (30 min), respectively. The elimination half-lives were 336 and 149 min for 10-gingerol and 6-shogaol, respectively. Thus, 10-gingerol and 6-shogaol were absorbed after per oral single dose of red ginger suspension and could be quantified in the plasma of the healthy subjects. Additionally, the red ginger analytes exhibited relatively slow elimination half-lives.
Keywords: Zingiber officinale var. Rubrum; Zingiberaceae; antiinflammation; gingerol; oleoresins; phenols; shogaol.
Figures
Similar articles
-
Pharmacokinetics and Tissue Distribution of Gingerols and Shogaols from Ginger (Zingiber officinale Rosc.) in Rats by UPLC⁻Q-Exactive⁻HRMS.Molecules. 2019 Jan 31;24(3):512. doi: 10.3390/molecules24030512. Molecules. 2019. PMID: 30708987 Free PMC article.
-
Liquid chromatographic determination of 6-, 8-, 10-gingerol, and 6-shogaol in ginger (Zingiber officinale) as the raw herb and dried aqueous extract.J AOAC Int. 2007 Sep-Oct;90(5):1219-26. J AOAC Int. 2007. PMID: 17955965
-
Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology.BMC Complement Altern Med. 2015 Jul 30;15:258. doi: 10.1186/s12906-015-0718-0. BMC Complement Altern Med. 2015. PMID: 26223685 Free PMC article.
-
Ginger from Farmyard to Town: Nutritional and Pharmacological Applications.Front Pharmacol. 2021 Nov 26;12:779352. doi: 10.3389/fphar.2021.779352. eCollection 2021. Front Pharmacol. 2021. PMID: 34899343 Free PMC article. Review.
-
Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes.Pharmaceuticals (Basel). 2021 Jun 15;14(6):571. doi: 10.3390/ph14060571. Pharmaceuticals (Basel). 2021. PMID: 34203813 Free PMC article. Review.
Cited by
-
Possible Use of Phytochemicals for Recovery from COVID-19-Induced Anosmia and Ageusia.Int J Mol Sci. 2021 Aug 18;22(16):8912. doi: 10.3390/ijms22168912. Int J Mol Sci. 2021. PMID: 34445619 Free PMC article. Review.
-
Impact of Thermal Processing on the Selected Biological Activities of Ginger Rhizome-A Review.Molecules. 2023 Jan 3;28(1):412. doi: 10.3390/molecules28010412. Molecules. 2023. PMID: 36615602 Free PMC article. Review.
-
6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential.Nutrients. 2019 Sep 28;11(10):2306. doi: 10.3390/nu11102306. Nutrients. 2019. PMID: 31569368 Free PMC article.
-
The therapeutic potential of naturally occurring 6-shogaol: an updated comprehensive review.Inflammopharmacology. 2025 Jun 18. doi: 10.1007/s10787-025-01812-z. Online ahead of print. Inflammopharmacology. 2025. PMID: 40531275 Review.
-
Zingiber officinale var. rubrum: Red Ginger's Medicinal Uses.Molecules. 2022 Jan 25;27(3):775. doi: 10.3390/molecules27030775. Molecules. 2022. PMID: 35164040 Free PMC article. Review.
References
-
- Zhang X, Iwaoka WT, Huang AS, Nakamoto ST, Wong R. Gingerol decreases after processing and storage of ginger. J Food Sci. 1994;59:1338–1340. doi: 10.1111/j.1365-2621.1994.tb14710.x. - DOI
-
- Hiserodt RD, Franzblau SG, Rosen RT. Isolation of 6-, 8-, and 10-gingerol from ginger rhizome by HPLC and preliminary evaluation of inhibition of Mycobacterium avium and Mycobacterium tuberculosis. J Agric Food Chem. 1998;46:2504–2508. doi: 10.1021/jf970948l. - DOI
-
- Young HY, Chiang CT, Huang YL, Pan FP, Chen GL. Analytical and stability studies of ginger preparations. Yao Wu Shi Pin Fen Xi. 2002;10:149–153.
-
- Riaz H, Begum A, Raza SA, Khan ZMUD, Yousaf H, Tariq A. Antimicrobial property and phytochemical study of ginger found in local area of Punjab, Pakistan. Int Curr Pharm J. 2015;4:405–409. doi: 10.3329/icpj.v4i7.23591. - DOI
-
- Ugwoke CEC, Nzekwe U. Phytochemistry and proximate composition of ginger (Zingiber officinale) J Pharm Allied Sci. 2010;7 No. 5.
LinkOut - more resources
Full Text Sources
