Pathological complete response to mFOLFOX6 plus cetuximab therapy for unresectable colon cancer with multiple paraaortic lymph node metastases
- PMID: 30546885
- PMCID: PMC6256093
- DOI: 10.3892/mco.2018.1742
Pathological complete response to mFOLFOX6 plus cetuximab therapy for unresectable colon cancer with multiple paraaortic lymph node metastases
Abstract
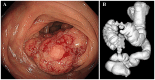
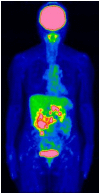
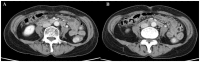
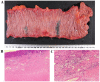
Pathological complete response is achievable with mFOLFOX6 plus cetuximab therapy for unresectable colorectal cancer with multiple paraaortic lymph node metastases (mCRC) despite right-sided colonic origin. A 62-year-old woman with synchronous paraaortic lymph node metastases of transverse colon cancer was treated with mFOLFOX6 plus cetuximab as first-line therapy. The tumor size was markedly decreased following 6 courses of chemotherapy, and all lymph node metastases had disappeared. The patient then underwent conventional right hemicolectomy with D3 lymph node dissection plus sampling excision of the paraaortic lymph nodes. The pathological diagnosis was a complete response. The patient is currently alive 5 years after surgery with no signs of recurrence. The present study reported the apparent effectiveness of conversion therapy (surgery) with combination treatment with mFOLFOX6 plus cetuximab and radical surgery. We hypothesized that patients with different types of mCRC of right-sided colon origin may be effectively treated with anti-EGFR monoclonal antibodies.
Keywords: colon cancer; conversion surgery; neoadjuvant chemotherapy; pathological complete response.
Figures
Similar articles
-
Pathologic Complete Response and Long-Term Survival After Preoperative Chemotherapy for Transverse Colon Cancer With Para-Aortic Lymph Node Metastases.Cureus. 2024 Apr 30;16(4):e59363. doi: 10.7759/cureus.59363. eCollection 2024 Apr. Cureus. 2024. PMID: 38689672 Free PMC article.
-
[Curative dissection of paraaortic lymph node metastases after operation for ascending colon cancer].Gan To Kagaku Ryoho. 2014 Nov;41(12):1611-3. Gan To Kagaku Ryoho. 2014. PMID: 25731270 Japanese.
-
Patients with RAS wild-type right-sided unresectable liver-confined mCRC also benefit from cetuximab plus chemotherapy in first-line treatment.Am J Cancer Res. 2018 Nov 1;8(11):2337-2345. eCollection 2018. Am J Cancer Res. 2018. PMID: 30555748 Free PMC article.
-
[Resection of Paraaortic Lymph Node Recurrence Wherein Complete Response to Bevacizumab Was Observed after Surgery for Sigmoid Colon Cancer].Gan To Kagaku Ryoho. 2016 Nov;43(12):1730-1732. Gan To Kagaku Ryoho. 2016. PMID: 28133113 Review. Japanese.
-
[A case of advanced sigmoid colon cancer with metastases in the liver and the paraaortic lymph nodes successfully treated with 5-FU/l-LV and FOLFOX4 followed by S-1 leading to long-term complete response].Gan To Kagaku Ryoho. 2014 Jan;41(1):113-6. Gan To Kagaku Ryoho. 2014. PMID: 24423964 Review. Japanese.
Cited by
-
Pathologic Complete Response and Long-Term Survival After Preoperative Chemotherapy for Transverse Colon Cancer With Para-Aortic Lymph Node Metastases.Cureus. 2024 Apr 30;16(4):e59363. doi: 10.7759/cureus.59363. eCollection 2024 Apr. Cureus. 2024. PMID: 38689672 Free PMC article.
-
Peritoneal dissemination of ascending colon cancer demonstrating relapse-free survival for 40 months with panitumumab monotherapy: A case report.Int J Surg Case Rep. 2019;59:41-45. doi: 10.1016/j.ijscr.2019.05.001. Epub 2019 May 9. Int J Surg Case Rep. 2019. PMID: 31103951 Free PMC article.
-
A Case of Complete Remission in Proficient Mismatch Repair (pMMR) Advanced Colon Cancer Treated with Sintilimab and XELOX.Immunotargets Ther. 2023 Feb 19;12:17-23. doi: 10.2147/ITT.S393526. eCollection 2023. Immunotargets Ther. 2023. PMID: 36844460 Free PMC article.
References
-
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. - DOI - PubMed
-
- Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. - DOI - PubMed
-
- De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. doi: 10.1001/jama.2010.1535. - DOI - PubMed
-
- Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
