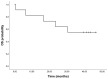
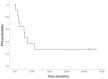
Trabectedin in combination with pegylated liposomal doxorubicin in patients with ovarian tumors
- PMID: 30546893
- PMCID: PMC6256111
- DOI: 10.3892/mco.2018.1729
Trabectedin in combination with pegylated liposomal doxorubicin in patients with ovarian tumors
Abstract
The majority of patients with ovarian cancer will experience relapse and thus require second-line therapy. While platinum-based therapies are the primary treatments for refractory disease other options are required, particularly for those with partially platinum-sensitive disease as their response rates are lower. Agents that can resensitize relapsed ovarian cancers to platinum, including trabectedin, are therefore of increasing interest. Trabectedin is a multitarget agent that has a complex, novel mechanism of action and has exhibited promising results in platinum-sensitive ovarian cancer when in combination with pegylated liposomal doxorubicin (PLD). The present study conducted retrospective analysis involving 11 cases (median age 60 years; range 45-75 years) of recurrent ovarian tumors and partial platinum sensitivity undergoing treatment with trabectedin + PLD. The cohort consisted of 7 serous carcinomas, 1 endometrial carcinoma, 2 undifferentiated carcinomas, and 1 mucinous carcinoma. Of the 11 patients, 4 exhibited a complete response, 3 achieved stable disease, and 4 had progression of disease. Mean overall survival was 32.42 months and median progression-free survival was 5.9 months. Trabectedin in combination with PLD was well tolerated in terms of gastrointestinal and hematological toxicity; Grade 3 cutaneous toxicity and grade 3 neutropenia were each observed in 18.2% of patients. There were no grade 4 events. Thus, the present study supports the use of trabectedin + PLD in patients with relapsed ovarian cancer and partial platinum sensitivity, with predictable and manageable toxicity.
Keywords: ovarian cancer; pegylated liposomal doxorubicin; recurrence; retrospective analysis; survival; trabectedin; treatment.
Figures
Similar articles
-
Multicenter retrospective study to evaluate the impact of trabectedin plus pegylated liposomal doxorubicin on the subsequent treatment in women with recurrent, platinum-sensitive ovarian cancer.Anticancer Drugs. 2019 Jul;30(6):628-635. doi: 10.1097/CAD.0000000000000794. Anticancer Drugs. 2019. PMID: 31008727
-
Retrospective multicenter study of elderly patients with platinum-sensitive relapsed ovarian cancer treated with trabectedin and pegylated liposomal doxorubicin (pld) in a real-world setting: a geico study.BMC Cancer. 2024 Jul 5;24(1):803. doi: 10.1186/s12885-024-12577-z. BMC Cancer. 2024. PMID: 38970024 Free PMC article.
-
GINECO Prospective Non-interventional PROSPECTYON Study: Trabectedin Plus Pegylated Liposomal Doxorubicin for Platinum-sensitive Recurrent Ovarian Cancer.Anticancer Res. 2020 Jul;40(7):3939-3945. doi: 10.21873/anticanres.14385. Anticancer Res. 2020. PMID: 32620635
-
Combinational use of trabectedin and pegylated liposomal doxorubicin for recurrent ovarian cancer: a meta-analysis of phase III randomized controlled trials.Am J Transl Res. 2023 Dec 15;15(12):6675-6689. eCollection 2023. Am J Transl Res. 2023. PMID: 38186978 Free PMC article. Review.
-
Recurrent ovarian cancer 8 months after induction and bevacizumab consolidation: rationale for using trabectedin + pegylated liposomal doxorubicin in second line.Expert Rev Anticancer Ther. 2018 Oct;18(sup1):13-17. doi: 10.1080/14737140.2018.1513792. Expert Rev Anticancer Ther. 2018. PMID: 30223694 Review.
References
LinkOut - more resources
Full Text Sources