Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma
- PMID: 30546944
- PMCID: PMC6287893
- DOI: 10.1080/2162402X.2018.1507669
Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma
Abstract
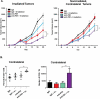
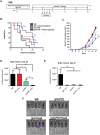
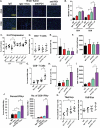
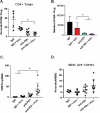
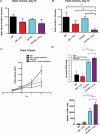
Nearly half of melanoma patients develop brain metastases during the course of their disease. Despite advances in both localized radiation and systemic immunotherapy, brain metastases remain difficult to treat, with most patients surviving less than 5 months from the time of diagnosis. While both treatment regimens have individually shown considerable promise in treating metastatic melanoma, there is interest in combining these strategies to take advantage of potential synergy. In order to study the ability of local radiation and anti-PD-1 immunotherapy to induce beneficial anti-tumor immune responses against distant, unirradiated tumors, we used two mouse models of metastatic melanoma in the brain, representing BRAF mutant and non-mutant tumors. Combination treatments produced a stronger systemic anti-tumor immune response than either treatment alone. This resulted in reduced tumor growth and larger numbers of activated, cytotoxic CD8+ T cells, even in the unirradiated tumor, indicative of an abscopal effect. The immune-mediated effects were present regardless of BRAF status. These data suggest that irradiation of brain metastases and anti-PD-1 immunotherapy together can induce abscopal anti-tumor responses that control both local and distant disease.
Keywords: Melanoma; antibody therapy; immunomodulation; immunotherapy; radiotherapy.
Figures
Similar articles
-
Irradiation enhanced the effects of PD-1 blockade in brain metastatic osteosarcoma.J Bone Oncol. 2018 Jun 25;12:61-64. doi: 10.1016/j.jbo.2018.05.002. eCollection 2018 Sep. J Bone Oncol. 2018. PMID: 29992089 Free PMC article.
-
Radiotherapy-Mediated Immunomodulation and Anti-Tumor Abscopal Effect Combining Immune Checkpoint Blockade.Cancers (Basel). 2020 Sep 25;12(10):2762. doi: 10.3390/cancers12102762. Cancers (Basel). 2020. PMID: 32992835 Free PMC article. Review.
-
Deep abscopal response to radiotherapy and anti-PD-1 in an oligometastatic melanoma patient with unfavorable pretreatment immune signature.Cancer Immunol Immunother. 2020 Sep;69(9):1823-1832. doi: 10.1007/s00262-020-02587-8. Epub 2020 Apr 29. Cancer Immunol Immunother. 2020. PMID: 32350591 Free PMC article.
-
Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy.Oncoimmunology. 2014 May 14;3:e28780. doi: 10.4161/onci.28780. eCollection 2014. Oncoimmunology. 2014. PMID: 25083318 Free PMC article.
-
The abscopal effect: systematic review in patients with brain and spine metastases.Neurooncol Adv. 2022 Aug 19;4(1):vdac132. doi: 10.1093/noajnl/vdac132. eCollection 2022 Jan-Dec. Neurooncol Adv. 2022. PMID: 36199973 Free PMC article. Review.
Cited by
-
A Comprehensive Revision of Radiation Immunotherapy and the Abscopal Effect in Central Nervous System Metastases: Reassessing the Frontier.Curr Issues Mol Biol. 2024 Oct 2;46(10):11075-11085. doi: 10.3390/cimb46100658. Curr Issues Mol Biol. 2024. PMID: 39451538 Free PMC article. Review.
-
Research Progress and Existing Problems for Abscopal Effect.Cancer Manag Res. 2020 Aug 3;12:6695-6706. doi: 10.2147/CMAR.S245426. eCollection 2020. Cancer Manag Res. 2020. PMID: 32801902 Free PMC article. Review.
-
Radiotherapy activates autophagy to increase CD8+ T cell infiltration by modulating major histocompatibility complex class-I expression in non-small cell lung cancer.J Int Med Res. 2019 Aug;47(8):3818-3830. doi: 10.1177/0300060519855595. Epub 2019 Jun 12. J Int Med Res. 2019. PMID: 31187666 Free PMC article.
-
Irradiation combined with PD-L1-/- and autophagy inhibition enhances the antitumor effect of lung cancer via cGAS-STING-mediated T cell activation.iScience. 2022 Jun 30;25(8):104690. doi: 10.1016/j.isci.2022.104690. eCollection 2022 Aug 19. iScience. 2022. PMID: 35847556 Free PMC article.
-
Guidelines in the management of CNS tumors.J Neurooncol. 2021 Feb;151(3):345-359. doi: 10.1007/s11060-020-03530-8. Epub 2021 Feb 21. J Neurooncol. 2021. PMID: 33611702 Review.
References
-
- Spagnolo F, Picasso V, Lambertini M, Ottaviano V, Dozin B, Queirolo P.. Survival of patients with metastatic melanoma and brain metastases in the era of MAP-kinase inhibitors and immunologic checkpoint blockade antibodies: A systematic review. Cancer Treat Rev. 2016;45:38–45. doi:10.1016/j.ctrv.2016.03.003. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
