Virotherapy-recruited PMN-MDSC infiltration of mesothelioma blocks antitumor CTL by IL-10-mediated dendritic cell suppression
- PMID: 30546960
- PMCID: PMC6287797
- DOI: 10.1080/2162402X.2018.1518672
Virotherapy-recruited PMN-MDSC infiltration of mesothelioma blocks antitumor CTL by IL-10-mediated dendritic cell suppression
Abstract
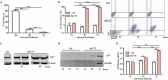
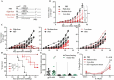
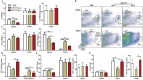
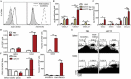
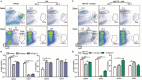
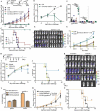
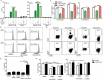
Antitumor cytotoxic T lymphocytes (CTLs) are essential for immune surveillance, yet the blockade of eliciting such CTLs during oncolytic virotherapy remains incompletely understood. Here, we show that oncolysis of mesothelioma by modified vaccinia Tiantan (MVTT) induces damage-associated molecular patterns exposure. Although MVTT leads to regression of established mesothelioma dose-dependently, antitumor CTLs are rarely induced. Mechanistically, MVTT virotherapy generates C-X-C chemokines that recruit CXCR2-expressing polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) into tumor microenvironment, where they suppress dendritic cells (DCs) by producing IL-10 and halt CTL responses. During the virotherapy, however, depletion of PMN-MDSCs but not of monocytic (M)-MDSCs results in the induction of potent antitumor CTLs that not only eradicate established mesothelioma but also prevent the second tumor challenge. Our findings suggest that vaccinia virotherapy may combine strategies that prevent the chemotactic recruitment of PMN-MDSCs, block their suppression on DCs or deplete PMN-MDSCs in order to induce potent CTLs for tumor eradication.
Keywords: CTLs; MDSCs; Modified vaccinia Tiantan; mesothelioma; virotherapy.
Figures
Similar articles
-
Enhancing the efficacy of vaccinia-based oncolytic virotherapy by inhibiting CXCR2-mediated MDSC trafficking.J Leukoc Biol. 2024 Mar 29;115(4):633-646. doi: 10.1093/jleuko/qiad150. J Leukoc Biol. 2024. PMID: 38066571
-
Visualization and quantification of in vivo homing kinetics of myeloid-derived suppressor cells in primary and metastatic cancer.Theranostics. 2019 Aug 12;9(20):5869-5885. doi: 10.7150/thno.33275. eCollection 2019. Theranostics. 2019. PMID: 31534525 Free PMC article.
-
Subsets of myeloid-derived suppressor cells in hepatocellular carcinoma express chemokines and chemokine receptors differentially.Int Immunopharmacol. 2015 Jun;26(2):314-21. doi: 10.1016/j.intimp.2015.04.010. Epub 2015 Apr 16. Int Immunopharmacol. 2015. PMID: 25891236
-
Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy.Oncoimmunology. 2015 Feb 3;4(1):e954829. doi: 10.4161/21624011.2014.954829. eCollection 2015 Jan. Oncoimmunology. 2015. PMID: 25949858 Free PMC article. Review.
-
Suppression of T cells by myeloid-derived suppressor cells in cancer.Hum Immunol. 2017 Feb;78(2):113-119. doi: 10.1016/j.humimm.2016.12.001. Epub 2016 Dec 7. Hum Immunol. 2017. PMID: 27939507 Review.
Cited by
-
Treatment with lentiviral vectors encoding shRNA against interleukin 10 modulates the immunosuppressive activity of murine colon carcinoma-associated myeloid-derived suppressor cells.Oncol Lett. 2021 Aug;22(2):582. doi: 10.3892/ol.2021.12843. Epub 2021 Jun 2. Oncol Lett. 2021. PMID: 34122633 Free PMC article.
-
Myeloid-intrinsic cell cycle-related kinase drives immunosuppression to promote tumorigenesis.iScience. 2023 Aug 12;26(10):107626. doi: 10.1016/j.isci.2023.107626. eCollection 2023 Oct 20. iScience. 2023. PMID: 37731616 Free PMC article.
-
Implications of immune cells in oncolytic herpes simplex virotherapy for glioma.Brain Tumor Pathol. 2022 Apr;39(2):57-64. doi: 10.1007/s10014-022-00431-8. Epub 2022 Apr 6. Brain Tumor Pathol. 2022. PMID: 35384530 Review.
-
Roles of N6-methyladenosine (m6A) modifications in gynecologic cancers: mechanisms and therapeutic targeting.Exp Hematol Oncol. 2022 Nov 12;11(1):98. doi: 10.1186/s40164-022-00357-z. Exp Hematol Oncol. 2022. PMID: 36371254 Free PMC article. Review.
-
Roles of the Exosomes Derived From Myeloid-Derived Suppressor Cells in Tumor Immunity and Cancer Progression.Front Immunol. 2022 Jan 27;13:817942. doi: 10.3389/fimmu.2022.817942. eCollection 2022. Front Immunol. 2022. PMID: 35154134 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
